Immunological instability of persistent adenovirus vectors in the brain: peripheral exposure to vector leads to renewed inflammation, reduced gene expression, and demyelination
- PMID: 8622134
- PMCID: PMC6579058
- DOI: 10.1523/JNEUROSCI.16-09-03045.1996
Immunological instability of persistent adenovirus vectors in the brain: peripheral exposure to vector leads to renewed inflammation, reduced gene expression, and demyelination
Abstract
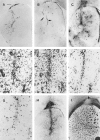
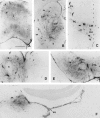
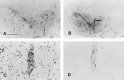
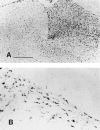
Nonreplicating adenovirus vectors are being developed as vehicles for the delivery of therapeutic genes in vivo. Whereas in many organs an antiviral T cell response eliminates the vector and damages local tissue, when adenovirus vectors are injected into the brain the subsequent immune attack can be ineffective, allowing the vector to persist. In the present study, E1-deleted human adenovirus vectors were injected into the caudate nucleus of rats. Two months later, expression of protein from the vector was still evident and little inflammation was seen. A subcutaneous injection of adenovirus vector at this time, however, led within 2 weeks to severe mononuclear inflammation and microglial activation in the caudate. This caused local demyelination and a decrease in detectable protein expression from the vector. Interestingly, intense microglial activation and numerous lymphocytes and monocytes were also seen in brain areas containing neurons capable of retrogradely transporting the adenovirus vector from the caudate. Control experiments established that this inflammation in distant brain areas was not a nonspecific consequence of degeneration. These experiments demonstrate that although adenovirus vectors can persist in the brain without causing chronic inflammation, they remain the potential target of a damaging cell-mediated immune response brought about by a subsequent peripheral exposure to vector. The finding of lymphocytes in brain areas that project to the caudate further shows that viral antigens that are retrogradely transported by neurons can also be the target of a T cell attack.
Figures

References
-
- Akli S, Caillaud C, Vigne E, Stratford-Perricaudet LD, Poenaru L, Perricaudet M, Kahn A, Pechanski MR. Transfer of a foreign gene into the brain using adenovirus vectors. Nature Genet. 1993;3:224–228. - PubMed
-
- Backes MG, Lund RD, Lagenaur CF, Kunz HW, Gill TJ. Cellular events associated with peripherally induced rejection of mature neural xenografts placed into neonatal rat brains. J Comp Neurol. 1990;295:428–437. - PubMed
-
- Bauer J, Sminia T, Wouterlood FG, Dijkstra CD. Phagocytic activity of macrophages and microglial cells during the course of acute and chronic relapsing experimental autoimmune encephalomyelitis. J Neurosci Res. 1994;38:365–375. - PubMed
-
- Berendse HW, Groenewegen HJ. Organization of the thalamostriatal projections in the rats, with special emphasis on the ventral striatum. J Comp Neurol. 1990;299:187–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
