Evolution of directional preferences in the supplementary eye field during acquisition of conditional oculomotor associations
- PMID: 8622136
- PMCID: PMC6579060
- DOI: 10.1523/JNEUROSCI.16-09-03067.1996
Evolution of directional preferences in the supplementary eye field during acquisition of conditional oculomotor associations
Abstract
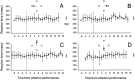
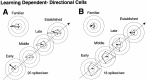
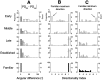
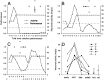
We assessed the preferred directions (PDs) of supplementary eye field (SEF) neurons during conditional visuomotor learning. Monkeys learned to select one of four saccadic eye movements in response to a foveal instruction stimulus (IS). ISs were either familiar or novel. Each familiar IS reliably evoked one saccade: 7 degrees left, right, up, or down form the central fixation point. Novel ISs initially triggered virtually random responses among those four possibilities, but the monkeys ultimately learned to select the instructed saccade. As reported previously, activity rates on novel IS trials significantly changed during learning. Some of these cells (learning-dependent) also have significant modulation on familiar IS trials, but others (learning-selective) lack such activity. Of the former, the familiar IS activity can be either directionally selective or omnidirectional. For most neurons, PDs were apparent during all phases of learning, but they were rarely constant. Only infrequently did a neuron's PD for novel ISs closely match that for familiar ISs throughout the learning process. In directional learning-dependent cells, the PD usually reoriented near the end of learning to resemble that for familiar IS trials. In omnidirectional cells, initially evident PDs dissipated with learning, even as the cell became more strongly modulated. Learning-selective cells typically began with significant PDs, but became unmodulated as learning progressed. Our findings show a pervasive lability in SEF PDs that may reflect a flexible and rapid remapping between inputs and responses within the premotor cortical network.
Figures







References
-
- Aizawa H, Inase M, Mushiake H, Shima K, Tanji J. Reorganization of activity in the supplementary motor area associated with motor learning and functional recovery. Exp Brain Res. 1991;84:668–671. - PubMed
-
- Ashe J, Georgopoulos AP. Movement parameters and neural activity in motor cortex and area 5. Cereb Cortex. 1994;6:590–600. - PubMed
-
- Ashe J, Taira M, Smyrnis N, Pellizzer G, Georgakopoulos T, Lurito JT, Georgopoulos AP. Motor cortical activity preceding a memorized movement trajectory with an orthogonal bend. Exp Brain Res. 1993;95:118–130. - PubMed
-
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. II. Spatial properties. J Neurophysiol. 1991;66:1109–1124. - PubMed
-
- Batschelet E. Academic; New York: 1981. Circular statistics in biology. .
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
