Mouse model of hyperkinesis implicates SNAP-25 in behavioral regulation
- PMID: 8622140
- PMCID: PMC6579059
- DOI: 10.1523/JNEUROSCI.16-09-03104.1996
Mouse model of hyperkinesis implicates SNAP-25 in behavioral regulation
Abstract
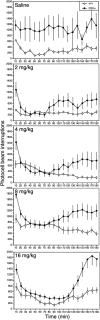
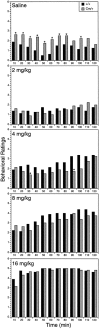
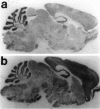
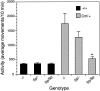
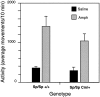
Although hyperkinesis is expressed in several neurological disorders, the biological basis of this phenotype is unknown. The mouse mutant coloboma (Cml+) exhibits profound spontaneous locomotor hyperactivity resulting from a deletion mutation. This deletion encompasses several genes including Snap, which encodes SNAP-25, a nerve terminal protein involved in neurotransmitter release. Administration of amphetamine, a drug that acts presynaptically, markedly reduced the locomotor activity in coloboma mice but increased the activity of control mice implicating presynaptic function in the behavioral abnormality. In contrast, the psychostimulant methylphenidate increased locomotor activity in both coloboma and control mice. When a transgene encoding SNAP-25 was bred into the coloboma strain to complement the Snap deletion, the hyperactivity expressed by these mice was rescued, returning these corrected mice to normal levels of locomotor activity. These results demonstrate that the hyperactivity exhibited by these mice is the result of abnormalities in presynaptic function specifically attributable to deficits in SNAP-25 expression.
Figures


References
-
- Anastopoulos AD, Barkley RA. Biological factors in attention deficit-hyperactivity disorder. Behav Ther. 1988;11:47–53.
-
- Arnold LE, Christopher J, Huestis R, Smeltzer DJ. Methylphenidate vs dextroamphetamine vs caffeine in minimal brain dysfunction. Arch Gen Psychiatry. 1978;35:463–473. - PubMed
-
- Bark IC. Structure of the chicken gene for SNAP-25 reveals duplicated exons encoding distinct isoforms of the protein. J Mol Biol. 1993;233:67–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
