Actin-based motility of isolated axoplasmic organelles
- PMID: 8635200
- PMCID: PMC4507568
- DOI: 10.1002/cm.970330202
Actin-based motility of isolated axoplasmic organelles
Abstract
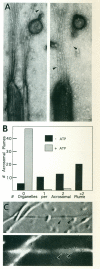
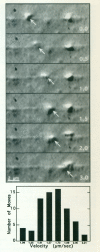
We previously showed that axoplasmic organelles from the squid giant axon move toward the barbed ends of actin filaments and that KI-washed organelles separated from soluble proteins by sucrose density fractionation retain a 235-kDa putative myosin. Here, we examine the myosin-like activities of KI-washed organelles after sucrose density fractionation to address the question whether the myosin on these organelles is functional. By electron microscopy KI-washed organelles bound to actin filaments in the absence of ATP but not in its presence. Analysis of organelle-dependent ATPase activity over time and with varying amounts of organelles revealed a basal activity of 350 (range: 315-384) nmoles Pi/mg/min and an actin-activated activity of 774 (range: 560-988) nmoles/mg/min, a higher specific activity than for the other fractions. By video microscopy washed organelles moved in only one direction on actin filaments with a net velocity of 1.11 +/- .03 microns/s and an instantaneous velocity of 1.63 +/- 0.29 microns/s. By immunogold electronmicroscopy, 7% of KI-washed organelles were decorated with an anti-myosin antibody as compared to 0.5% with non-immune serum. Thus, some axoplasmic organelles have a tightly associated myosin-like activity.
Figures

References
-
- Allen RD, Metuzals J, Tasaki I, Brady ST, Gilbert SP. Fast axonal transport in squid giant axon. Science. 1982;218:1127–1128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

