Tau is enriched on dynamic microtubules in the distal region of growing axons
- PMID: 8642405
- PMCID: PMC6578833
- DOI: 10.1523/JNEUROSCI.16-11-03601.1996
Tau is enriched on dynamic microtubules in the distal region of growing axons
Abstract
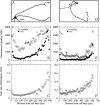
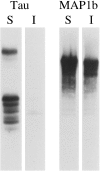
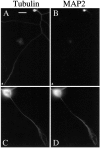
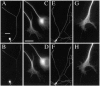
It is widely held that tau determines the stability of microtubules in growing axons, although direct evidence supporting this hypothesis is lacking. Previous studies have shown that the microtubule polymer in the distal axon and growth cone is the most dynamic of growing axons; it turns over more rapidly and is more sensitive to microtubule depolymerizing drugs than the polymer situated proximally. We reasoned that if the stability of axonal microtubules is directly related to their content of tau, then the polymer in the distal axon should have less tau than the polymer in the proximal axon. We tested this proposition by measuring the relative tau content of microtubule along growing axons of cultured sympathetic neurons immunostained for tau and tubulin. Our results show that the tau content of microtubules varies along the axon, but in the opposite way predicted. Specifically, the relative tau content of microtubules increases progressively along the axon to reach a peak near the growth cone that is severalfold greater than that observed proximally. Thus, tau is most enriched on the most dynamic polymer of the axon. We also show that the gradient in tau content of microtubules does not generate corresponding gradients in the extent of tubulin assembly or in the sensitivity of axonal microtubules to nocodazole. On the basis of these findings, we propose that tau in growing axons has functions other than promoting microtubule assembly and stability and the key sites for these functions are the distal axon and growth cone.
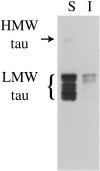
Figures







References
-
- Baas PW, Pienkowski TP, Cimbalnik KA, Toyama K, Bakalis S, Ahmad FJ, Kosik KS. Tau confers drug-stability but not cold-stability to microtubules in living cells. J Cell Sci. 1994;107:135–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
