Determinants of competitive antagonist sensitivity on neuronal nicotinic receptor beta subunits
- PMID: 8656274
- PMCID: PMC6578601
- DOI: 10.1523/JNEUROSCI.16-12-03798.1996
Determinants of competitive antagonist sensitivity on neuronal nicotinic receptor beta subunits
Abstract
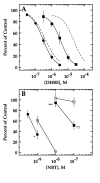
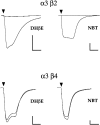
We constructed a series of chimeric and mutant neuronal nicotinic acetylcholine receptor beta subunits to map amino acid residues that determine sensitivity to competitive antagonists. The beta 2 and beta 4 subunits form pharmacologically distinct receptors when expressed in combination with the alpha 3 subunit in Xenopus oocytes. At equipotent acetylcholine concentrations, alpha 3 beta 2 is 56-fold more sensitive to blockage by dihydro-beta-erythroidine than is alpha 3 beta 4. The alpha 3 beta 2 combination is also sensitive to long-term blockade by neuronal bungarotoxin, whereas alpha 3 beta 4 is not. Pharmacological analysis of receptors formed by chimeric beta subunits reveals that amino acid residues that determine both dihydro-beta-erythroidine and neuronal bungarotoxin sensitivity are located within several sequence segments. The major determinant of sensitivity to both competitive antagonists is located between residues 54 and 63. A minor determinant of sensitivity to both antagonists lies between residues 1 and 54, whereas a minor determinant of NBT sensitivity lies between residues 74 and 80. Within region 54-63 of beta 2, mutant beta 2 subunits were used to identify threonine 59 as a residue critical in determining competitive antagonist sensitivity. Changing threonine 59 to lysine, as occurs in beta 4, causes a 9-fold decrease in dihydro-beta-erythroidine sensitivity and a 71-fold decrease in neuronal bungarotoxin sensitivity. Changing polar threonine 59 to negatively charged aspartate causes a 2.5-fold increase in neuronal bungarotoxin sensitivity and has no effect on dihydro-beta-erythroidine sensitivity.
Figures



References
-
- Abramson SN, Li Y, Culver P, Taylor P. An analog of lophotoxin reacts covalently with Tyr190 in the α-subunit of the nicotinic acetylcholine receptor. J Biol Chem. 1989;264:12666–12672. - PubMed
-
- Bertrand D, Bertrand S, Ballivet M. Pharmacological properties of the homomeric α7 receptor. Neurosci Lett. 1992;146:87–90. - PubMed
-
- Blount P, Merlie J. Molecular basis of the two nonequivalent ligand binding sites of the muscle nicotinic acetylcholine receptor. Neuron. 1989;3:349–357. - PubMed
-
- Cohen JB, Blanton MP, Chiara DC, Sharp SD, White BH. Structural organization of functional domains of the nicotinic acetylcholine receptor. J Cell Biochem [Suppl] 1992;16E:217.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
