Muscarinic activation of a voltage-dependent cation nonselective current in rat association cortex
- PMID: 8656279
- PMCID: PMC6578620
- DOI: 10.1523/JNEUROSCI.16-12-03848.1996
Muscarinic activation of a voltage-dependent cation nonselective current in rat association cortex
Abstract
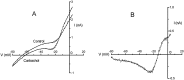
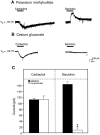
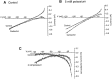
The ionic mechanism underlying the acetylcholine-induced depolarization of layer V pyramidal neurons of rat prefrontal cortex was examined using whole-cell recording in in vitro rat brain slices. Consistent with previous results, pressure application of acetylcholine to layer V pyramidal neurons elicited a strong depolarization. Pharmacological analysis of this response indicated that it was mediated by the stimulation of muscarinic receptors as it was mimicked by muscarinic agonists, but not by nicotine, and was blocked by atropine. The inward current responsible for the depolarization resulted from the activation of a voltage-dependent, cation nonselective current. Thus, the amplitude of the current was critically dependent on extracellular sodium concentration but not on extracellular potassium or chloride concentration. Examination of the I-V relationship for the muscarinic current using voltage clamp revealed that the current reversed near -15 mV and exhibited a strong voltage dependence, turning off rapidly in the subthreshold range. The voltage dependence of the current led to the appearance of a current associated with a conductance decrease when examined using steady-state voltage- or current-clamp measurements. This might have led to earlier misidentification of this response as mediated by a decrease in potassium conductance. These results question the traditional interpretation that muscarinic depolarization in cortex is mediated by a decrease in potassium conductance. They indicate that the fundamental mechanism responsible for muscarinic depolarization in prefrontal cortex involves the activation of a voltage-dependent, cation nonselective current. This current might represent a previously unsuspected mechanism capable of mediating slow depolarization in the central nervous system.
Figures









References
-
- Andrade R. Cell excitation enhances muscarinic cholinergic responses in rat association cortex. Brain Res. 1991;548:81–93. - PubMed
-
- Araneda R, Andrade R. 5-HT2 and 5-HT1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. - PubMed
-
- Benardo LS, Prince DA. Ionic mechanisms of cholinergic excitation in mammalian hippocampal pyramidal cells. Brain Res. 1982;249:333–344. - PubMed
-
- Benham CD. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985;316:345–346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
