Homeostasis of synaptic transmission in Drosophila with genetically altered nerve terminal morphology
- PMID: 8656281
- PMCID: PMC6578607
- DOI: 10.1523/JNEUROSCI.16-12-03877.1996
Homeostasis of synaptic transmission in Drosophila with genetically altered nerve terminal morphology
Abstract
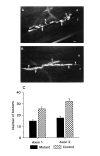
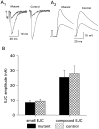
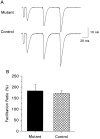
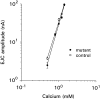
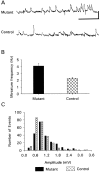
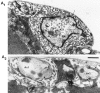
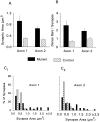
We present a new test of the hypothesis that synaptic strength is directly related to nerve terminal morphology through analysis of synaptic transmission at Drosophila neuromuscular junctions with a genetically reduced number of nerve terminal varicosities. Synaptic transmission would decrease in target cells with fewer varicosities if there is a relationship between the number of varicosities and the strength of synaptic transmission. Animals that have an extreme hypomorphic allele of the gene for the cell adhesion molecule Fasciclin II possess fewer synapse-bearing nerve terminal varicosities; nevertheless, synaptic strength is maintained at a normal level for the muscle cell as a whole. Fewer failures of neurotransmitter release and larger excitatory junction potentials from individual varicosities, as well as more frequent spontaneous release and larger quantal units, provide evidence for enhancement of transmitter release from varicosities in the mutant. Ultrastructural analysis reveals that mutant nerve terminals have bigger synapses with more active zones per synapse, indicating that synaptic enlargement and an accompanying increase in synaptic complexity provide for more transmitter release at mutant varicosities. These results show that morphological parameters of transmitting nerve terminals can be adjusted to functionally compensate for genetic perturbations, thereby maintaining optimal synaptic transmission.
Figures


References
-
- Atwood HL, Govind CK, Wu C-F. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993;24:1008–1024. - PubMed
-
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. - PubMed
-
- Connor EA, Smith MA. Retrograde signalling in the formation and maintenance of the neuromuscular junction. J Neurobiol. 1994;25:722–739. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
