Motor neurons are selectively vulnerable to AMPA/kainate receptor-mediated injury in vitro
- PMID: 8753869
- PMCID: PMC6578994
- DOI: 10.1523/JNEUROSCI.16-13-04069.1996
Motor neurons are selectively vulnerable to AMPA/kainate receptor-mediated injury in vitro
Abstract
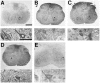
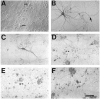
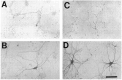
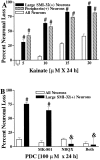
The nonphosphorylated neurofilament marker SMI-32 stains motor neurons in spinal cord slices and stains a subset of cultured spinal neurons ["large SMI-32(+) neurons"], which have a morphology consistent with motor neurons identified in vitro: large cell body, long axon, and extensive dendritic arborization. They are found preferentially in ventral spinal cord cultures, providing further evidence that large SMI-32(+) neurons are indeed motor neurons, and SMI-32 staining often colocalizes with established motor neuron markers (including acetylcholine, calcitonin gene-related peptide, and peripherin). Additionally, choline acetyltransferase activity (a frequently used index of the motor neuron population) and peripherin(+) neurons share with large SMI-32(+) neurons an unusual vulnerability to AMPA/kainate receptor-mediated injury. Kainate-induced loss of these motor neuron markers is Ca2+-dependent, which supports a critical role of Ca2+ ions in this injury. Raising extracellular Ca2+ exacerbates injury, whereas removal of extracellular Ca2+ is protective. A basis for this vulnerability is provided by the observation that most peripherin(+) neurons, like large SMI-32(+) neurons, are subject to kainate-stimulated Co2+ uptake, a histochemical stain that identifies neurons possessing Ca2+-permeable AMPA/kainate receptor-gated channels. Finally, of possibly greater relevance to the slow motor neuronal degeneration in diseases, both large SMI-32(+) neurons and peripherin(+) neurons are selectively damaged by prolonged (24 hr) low-level exposures to kainate (10 microM) or to the glutamate reuptake blocker L-trans-pyrrolidine-2,4-dicarboxylic acid (100 microM). During these low-level kainate exposures, large SMI-32(+) neurons showed higher intracellular Ca2+ concentrations than most spinal neurons, suggesting that Ca2+ ions are also important in this more slowly evolving injury.
Figures



References
-
- Ang LC, Bhaumich B, Munoz DG, Sass J, Juurlink BHJ. Effects of astrocytes, insulin and insulin-like growth factor 1 on the survival of motor neurons in vitro . J Neurol Sci. 1992;109:168–172. - PubMed
-
- Bochet P, Audinet E, Lambolez B, Crepel F, Rossier J, Iino M, Tsuzuki K, Ozawa S. Subunit composition at the single-cell level explains functional properties of a glutamate-gated channel. Neuron. 1994;12:383–388. - PubMed
-
- Brorson JR, Bleakman D, Chard PS, Miller RJ. Calcium directly permeates kainate/alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptors in cultured cerebellar Purkinje neurons. Mol Pharmacol. 1992;41:603–608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
