Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing bcl-2
- PMID: 8753880
- PMCID: PMC6578989
- DOI: 10.1523/JNEUROSCI.16-13-04186.1996
Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing bcl-2
Abstract
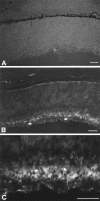
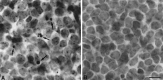
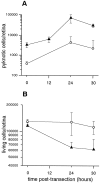
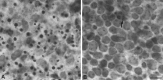
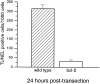
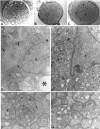
Approximately half of the retinal ganglion cells (RGCs) present in the rodent retina at birth normally die during early development. Overexpression of the photo-oncogene bcl-2 recently has been shown to rescue some neuronal populations from natural cell death and from degeneration induced by axotomy of nerves within the peripheral nervous system. Here we study in vivo the role of the overexpression of bcl-2 in the natural cell death of RGCs and in the degenerative process induced in these cells by transection of the optic nerve. We find that in newborn bcl-2 transgenic mice, the number of RGCs undergoing natural cell death is considerably lower than in wild-type pups. Consistently, a vast majority (90%) of the ganglion cells found in the retina of neonatal transgenics are maintained in adulthood, whereas only 40% survive in wild-type mice. After transection of the optic nerve, the number of degenerating ganglion cells, determined by counting pyknotic nuclei or nuclei with fragmented DNA, is substantially reduced in transgenic mice. In wild-type animals, almost 50% of ganglion cells degenerate in the 24 hr after the lesion, whereas almost the entire ganglion cell population survives axotomy in transgenic mice. Therefore, overexpression of bcl-2 is effective in preventing degeneration of this neuronal population, raising the possibility that ganglion cells are dependent on the endogenous expression of bcl-2 for survival. The remarkable rescue capacity of bcl-2 overexpression in these neurons makes it an interesting model for studying natural cell death and responses to injury in the CNS.
Figures

References
-
- Allsopp TE, Kiselov S, Wyatt S, Davies AM. Role of bcl-2 in the brain-derived neurotrophic factor survival response. Eur J Neurosci. 1995;7:1266–1272. - PubMed
-
- Allsopp TE, Wyatt S, Patterson HF, Davies AM. The proto-oncogene bcl-2 can selectively rescue neurotrophic factor-dependent neurons from apoptosis. Cell. 1993;73:295–307. - PubMed
-
- Beazley LD, Perry VH, Baker B, Darby EJ. An investigation into the role of ganglion cells in the regulation of division and death of other retinal cells. Dev Brain Res. 1987;33:169–184. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
