Voltage-gated K+ channel beta subunits: expression and distribution of Kv beta 1 and Kv beta 2 in adult rat brain
- PMID: 8756417
- PMCID: PMC6579302
- DOI: 10.1523/JNEUROSCI.16-16-04846.1996
Voltage-gated K+ channel beta subunits: expression and distribution of Kv beta 1 and Kv beta 2 in adult rat brain
Abstract
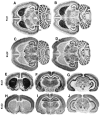
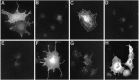
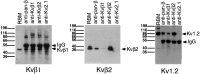
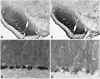
Recent cloning of K+ channel beta subunits revealed that these cytoplasmic polypeptides can dramatically alter the kinetics of current inactivation and promote efficient glycosylation and surface expression of the channel-forming alpha subunits. Here, we examined the expression, distribution, and association of two of these beta subunits, Kv beta 1 and Kv beta 2, in adult rat brain. In situ hybridization using cRNA probes revealed that these beta-subunit genes are heterogeneously expressed, with high densities of Kv beta 1 mRNA in the striatum, CA1 subfield of the hippocampus, and cerebellar Purkinje cells, and high densities of Kv beta 2 mRNA in the cerebral cortex, cerebellum, and brainstem. Immunohistochemical staining using subunit-specific monoclonal and affinity-purified polyclonal antibodies revealed that the Kv beta 1 and Kv beta 2 polypeptides frequently co-localize and are concentrated in neuronal perikarya, dendrites, and terminal fields, and in the juxtaparanodal region of myelinated axons. Immunoblot and reciprocal co-immunoprecipitation analyses indicated that Kv beta 2 is the major beta subunit present in rat brain membranes, and that most K+ channel complexes containing Kv beta 1 also contain Kv beta 2. Taken together, these data suggest that Kv beta 2 is a component of almost all K+ channel complexes containing Kv 1 alpha subunits, and that individual channels may contain two or more biochemically and functionally distinct beta-subunit polypeptides.
Figures



References
-
- Arai M, Prystowsky MB, Cohen JA. Expression of the T-lymphocyte activation gene, F5, by mature neurons. J Neurosci Res. 1992;33:527–537. - PubMed
-
- Cohen JA, Arai M, Luning Prak E, Brooks SA, Young LH, Prystowsky MB. Characterization of a novel mRNA expressed by neurons in mature brain. J Neurosci Res. 1992;31:273–284. - PubMed
-
- England SK, Uebele VN, Kodali J, Bennett PB, Tamkun MM. A novel K+ channel β-subunit (hKvβ1.3) is produced via alternative mRNA splicing. J Biol Chem. 1995a;270:28531–28534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
