Actions of substance P on rat neostriatal neurons in vitro
- PMID: 8756443
- PMCID: PMC6579311
- DOI: 10.1523/JNEUROSCI.16-16-05141.1996
Actions of substance P on rat neostriatal neurons in vitro
Abstract
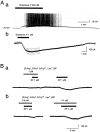
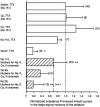
Actions of substance P (SP) on the neostriatal neurons in in vitro rat slice preparations were studied via whole-cell patch-clamp recording. Almost all large aspiny neurons (cholinergic cells) and half of the low-threshold spike (LTS) cells (somatostatin/ NOS-positive cells) showed depolarization or an inward shift of the holding currents in response to bath-applied SP in a dose-dependent manner. In contrast, no responses were observed in fast-spiking (FS) cells (parvalbumin-positive cells) and medium spiny cells. Spike discharges followed by slow EPSPs/EPSCs were evoked by intrastriatal electrical stimulation in the large aspiny neurons. Pretreatment with [D-Arg1, D-Pro2, D-Trp7,9, Leu11]-SP, an antagonist of the SP receptor, reversibly suppressed the induction of the slow EPSPs/EPSCs and unmasked slow IPSCs. The SP-induced inward current, although almost unchanged even after the blockade of Ih channels and voltage-dependent Na+, Ca2+, and K+ channels, changed its amplitude according to the Na+ concentration used in both the large aspiny neurons and LTS cells. Thus, the cation current could account for virtually all of the inward current at resting levels in both neurons. These results suggest that the firing of afferent neurons such as striatonigral medium spiny neurons, one of the possible sources of SP, would increase the firing probability of the two types of interneurons of the neostriatum by SP-receptor-mediated opening of tetrodotoxin-insensitive cation channels.
Figures








References
-
- Akins PT, Surmeier DJ, Kitai ST. Muscarinic modulation of a transient K+ conductance in rat neostriatal neurons. Nature. 1990;344:240–242. - PubMed
-
- Anderson JJ, Chase TN, Engber TM. Substance P increases release of acetylcholine in the dorsal striatum of freely moving rats. Brain Res. 1993;623:189–194. - PubMed
-
- Aosaki T, Kawaguchi Y. Actions of neuropeptides on large aspiny neurons of rat neostriatum in vitro. Soc Neurosci Abstr. 1995;21:913.
-
- Aosaki T, Graybiel AM, Kimura M. Effects of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994a;265:412–415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
