Gonadotropin-releasing hormone and NMDA receptor gene expression and colocalization change during puberty in female rats
- PMID: 8757241
- PMCID: PMC6578900
- DOI: 10.1523/JNEUROSCI.16-17-05281.1996
Gonadotropin-releasing hormone and NMDA receptor gene expression and colocalization change during puberty in female rats
Abstract
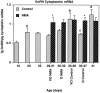
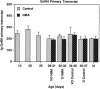
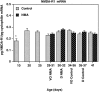
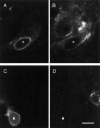
During development, an increase in gonadotropin-releasing hormone (GnRH) release occurs that is critical for the initiation of puberty. This increase is attributable, at least in part, to activation of the GnRH neurosecretory system by inputs from neurotransmitters, such as glutamate, acting via NMDA receptors. We examined changes in GnRH and NMDA-R1 gene expression by RNase protection assay of preoptic area-anterior hypothalamic (POA-AH) dissections of female rats undergoing normal puberty or in which precocious puberty was induced by treatment with the glutamate agonist NMA. GnRH mRNA levels increased significantly throughout normal development; this was accelerated by treatment with NMA. NMDA-R1 mRNA levels increased only between P10 and P20. The acceleration of the elevation in GnRH mRNA levels by NMDA suggests that a stimulation of GnRH gene expression may be a rate-limiting factor for the onset of puberty. This is attributable to a post-transcriptional mechanism because GnRH primary transcript levels, an index of proGnRH gene transcription, were not observed to change during puberty. Alterations in the colocalization of GnRH neurons with the NMDA-R1 subunit during puberty also were assessed immunocytochemically. The percentage of GnRH neurons that double-labeled with NMDA-R1 was 2% in prepubertal rats and 3% in pubertal rats; this increased to 19% in postpubertal rats. Taken together, these studies suggest that an increase in glutamatergic input to GnRH neurons plays a role in the increase in GnRH release and gene expression that occurs at the initiation of puberty.
Figures


Similar articles
-
Perinatal changes in hypothalamic N-methyl-D-aspartate receptors and their relationship to gonadotropin-releasing hormone neurons.Endocrinology. 1999 May;140(5):2288-96. doi: 10.1210/endo.140.5.6749. Endocrinology. 1999. PMID: 10218982
-
Age-related changes in hypothalamic gonadotropin-releasing hormone and N-methyl-D-aspartate receptor gene expression, and their regulation by oestrogen, in the female rat.J Neuroendocrinol. 2002 Apr;14(4):300-9. doi: 10.1046/j.1365-2826.2002.00777.x. J Neuroendocrinol. 2002. PMID: 11963827
-
Neuroendocrine aging in the female rat: the changing relationship of hypothalamic gonadotropin-releasing hormone neurons and N-methyl-D-aspartate receptors.Endocrinology. 2000 Dec;141(12):4757-67. doi: 10.1210/endo.141.12.7841. Endocrinology. 2000. PMID: 11108291
-
Gonadotropin-releasing hormone neurons, NMDA receptors, and their regulation by steroid hormones across the reproductive life cycle.Brain Res Brain Res Rev. 2001 Nov;37(1-3):235-48. doi: 10.1016/s0165-0173(01)00121-7. Brain Res Brain Res Rev. 2001. PMID: 11744089 Review.
-
Excitatory amino acids: function and significance in reproduction and neuroendocrine regulation.Front Neuroendocrinol. 1994 Mar;15(1):3-49. doi: 10.1006/frne.1994.1002. Front Neuroendocrinol. 1994. PMID: 7958168 Review.
Cited by
-
GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice.J Neurosci. 1999 Mar 15;19(6):2037-50. doi: 10.1523/JNEUROSCI.19-06-02037.1999. J Neurosci. 1999. PMID: 10066257 Free PMC article.
-
Central Precocious Puberty During the COVID-19 Pandemic Period: A Systematic Review of Literature.Cureus. 2024 Oct 7;16(10):e71002. doi: 10.7759/cureus.71002. eCollection 2024 Oct. Cureus. 2024. PMID: 39507164 Free PMC article. Review.
-
NMDA receptor subunit NR2b: effects on LH release and GnRH gene expression in young and middle-aged female rats, with modulation by estradiol.Neuroendocrinology. 2008;87(3):129-41. doi: 10.1159/000111136. Epub 2007 Nov 15. Neuroendocrinology. 2008. PMID: 18025808 Free PMC article.
-
The Changes They are A-Timed: Metabolism, Endogenous Clocks, and the Timing of Puberty.Front Endocrinol (Lausanne). 2012 Mar 28;3:45. doi: 10.3389/fendo.2012.00045. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 22645521 Free PMC article.
-
Regulatory role of excitatory amino acids in reproduction.Endocrine. 2005 Dec;28(3):271-80. doi: 10.1385/ENDO:28:3:271. Endocrine. 2005. PMID: 16388116 Review.
References
-
- Abbud R, Smith MS. Do GnRH neurons express the gene for the NMDA receptor? Brain Res. 1995;690:117–120. - PubMed
-
- Advis JP, Simpkins JW, Chen HT, Meites J. Relation of biogenic amines to onset of puberty in the female rat. Endocrinology. 1978;103:11–16. - PubMed
-
- Everett JW, Radford HM, Holsinger J. Electrolyte irritative lesions in the hypothalamus and other forebrain areas: effects on luteinizing hormone release and the ovarian cycle. In: Martini L, Pecile A, editors. Hormonal steroids. Academic; New York: 1964. pp. 235–254.
-
- Eyigor O, Jennes L. Immunocytochemical localization of gonadotropin-releasing hormone, glutamate, and kainate-2 receptor protein in rat brain. Soc Neurosci Abstr. 1995;21:1889.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
