Impaired differentiation of Schwann cells in transgenic mice with increased PMP22 gene dosage
- PMID: 8757248
- PMCID: PMC6578876
- DOI: 10.1523/JNEUROSCI.16-17-05351.1996
Impaired differentiation of Schwann cells in transgenic mice with increased PMP22 gene dosage
Abstract
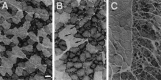
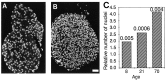
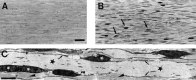
An intrachromosomal duplication containing the PMP22 gene is associated with the human hereditary peripheral neuropathy Charcot-Marie-Tooth disease type 1A, and PMP22 overexpression as a consequence of increased PMP22 gene dosage has been suggested as causative event in this frequent disorder of peripheral nerves. We have generated transgenic mice that carry additional copies of the pmp22 gene to prove that increased PMP22 gene dosage is sufficient to cause PNS myelin deficiencies. Mice carrying approximately 16 and 30 copies of the pmp22 gene display a severe congenital hypomyelinating neuropathy as characterized by an almost complete lack of myelin and marked slowing of nerve conductions. Affected nerves contain an increased number of nonmyelinating Schwann cells, which do not form onion bulbs but align in association with axons. The mutant Schwann cells are characterized by a premyelination-like state as indicated by the expression of embryonic Schwann cell markers. Furthermore, continued Schwann cell proliferation is observed into adulthood. We hypothesize that Schwann cells are impaired in their differentiation into the myelinating phenotype, leading to a disorder comparable to severe cases of hereditary motor and sensory neuropathies. Our findings, combined with the analysis of heterozygous and homozygous PMP22-deficient mice, indicate that aberrant pmp22 gene copy numbers cause various forms of myelination defects.
Figures

Similar articles
-
Schwann cell differentiation in Charcot-Marie-Tooth disease type 1A (CMT1A): normal number of myelinating Schwann cells in young CMT1A patients and neural cell adhesion molecule expression in onion bulbs.Acta Neuropathol. 1997 Oct;94(4):310-5. doi: 10.1007/s004010050712. Acta Neuropathol. 1997. PMID: 9341930
-
Distal axonopathy in peripheral nerves of PMP22-mutant mice.Brain. 1999 Aug;122 ( Pt 8):1563-77. doi: 10.1093/brain/122.8.1563. Brain. 1999. PMID: 10430839
-
Regulation of Schwann cell proliferation and apoptosis in PMP22-deficient mice and mouse models of Charcot-Marie-Tooth disease type 1A.Brain. 2001 Nov;124(Pt 11):2177-87. doi: 10.1093/brain/124.11.2177. Brain. 2001. PMID: 11673320
-
Regulation of myelin-specific gene expression. Relevance to CMT1.Ann N Y Acad Sci. 1999 Sep 14;883:91-108. Ann N Y Acad Sci. 1999. PMID: 10586235 Review.
-
Many facets of the peripheral myelin protein PMP22 in myelination and disease.Microsc Res Tech. 1998 Jun 1;41(5):359-71. doi: 10.1002/(SICI)1097-0029(19980601)41:5<359::AID-JEMT3>3.0.CO;2-L. Microsc Res Tech. 1998. PMID: 9672419 Review.
Cited by
-
Rho-dependent regulation of cell spreading by the tetraspan membrane protein Gas3/PMP22.Mol Biol Cell. 1999 Jul;10(7):2441-59. doi: 10.1091/mbc.10.7.2441. Mol Biol Cell. 1999. PMID: 10397775 Free PMC article.
-
Oligodendroglial response to the immune cytokine interferon gamma.Neurochem Res. 1999 Feb;24(2):331-8. doi: 10.1023/a:1022586726510. Neurochem Res. 1999. PMID: 9972883 Review.
-
Charcot-Marie-Tooth disease: lessons in genetic mechanisms.Mol Med. 1998 Jan;4(1):3-11. Mol Med. 1998. PMID: 9513184 Free PMC article. Review. No abstract available.
-
CMT1A current gene therapy approaches and promising biomarkers.Neural Regen Res. 2023 Jul;18(7):1434-1440. doi: 10.4103/1673-5374.361538. Neural Regen Res. 2023. PMID: 36571339 Free PMC article. Review.
-
gas7: A gene expressed preferentially in growth-arrested fibroblasts and terminally differentiated Purkinje neurons affects neurite formation.Proc Natl Acad Sci U S A. 1998 Sep 15;95(19):11423-8. doi: 10.1073/pnas.95.19.11423. Proc Natl Acad Sci U S A. 1998. PMID: 9736752 Free PMC article.
References
-
- Adlkofer K, Martini R, Aguzzi A, Zielasek J, Toyka KV, Suter U. Hypermyelination and demyelinating peripheral neuropathy in PMP22-deficient mice. Nature Genet. 1995;11:274–280. - PubMed
-
- Baechner D, Liehr T, Hameister H, Altenberger H, Grehl H, Suter U, Rautenstrauss B. Widespread expression of the peripheral myelin protein-22 gene (PMP22) in the neural and non-neural tissues during murine development. J Neurosci Res. 1995;42:735–741. - PubMed
-
- Bascles L, Bonnet J, Garbay B. Expression of the PMP22 gene in trembler mutant mice: comparison with the other myelin protein genes. Dev Neurosci. 1992;14:336–341. - PubMed
-
- Bermingham JRJ, O’Connell S, Arroyo E, Powell F, Kalla K, McEvilly R, Scherer SS, Rosenfeld MG. Mutation of the POU domain transcription factor TST-1/OCT6/SCIP in mice produces neuronal and myelinating Schwann cell defects. Soc Neurosci Abstr. 1995;21:5.
-
- Bunge RP, Bunge MB, Eldridge CF. Linkage between axonal ensheathment and basal lamina production by Schwann cells. Annu Rev Neurosci. 1986;9:305–328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
