Extracellular synthesis of cADP-ribose from nicotinamide-adenine dinucleotide by rat cortical astrocytes in culture
- PMID: 8757250
- PMCID: PMC6578884
- DOI: 10.1523/JNEUROSCI.16-17-05372.1996
Extracellular synthesis of cADP-ribose from nicotinamide-adenine dinucleotide by rat cortical astrocytes in culture
Abstract
cADPR is an endogenous calcium-mobilizing agent that in vertebrates is synthesized from nicotinamide-adenine dinucleotide (NAD) by bifunctional enzymes with ADP-ribosyl cyclase and cADPR hydrolase activity. ADP-ribosyl cyclase and cADPR hydrolase activity have been reported in the brain, but the cellular localization of these activities has not been determined previously. In the present study, selective culturing techniques were employed to localize ADP-ribosyl cyclase activity and cADPR hydrolase activity to astrocytes or neurons in cultures derived from rat embryonic cerebral cortex. ADP-ribosyl cyclase activity was determined by incubating cultures with 1 mM NAD in the extracellular medium for 60 min at 37 degrees C and measuring formation of cADPR by bioassay and by HPLC. Astrocyte cultures and mixed cultures of astrocytes and neurons had mean specific activities of 0.84 +/- 0.06 and 0.9 +/- 0.18 nmol cADPR produced/mg protein/hr, respectively. No detectable ADP-ribosyl cyclase activity was found in neuron-enriched/ astrocyte-poor cultures. cADPR hydrolase activity was detectable by incubating cultures with 300 microM cADPR for 60 min at 37 degrees C and assaying loss of cADPR or accumulation of ADPR. The demonstration of extracellular ADP-ribosyl cyclase and cADPR hydrolase activities associated with astrocytes may have important implications for the role of extracellular cADPR in signal transduction and in intercellular communication in the nervous system.
Figures

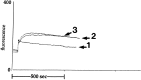
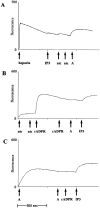


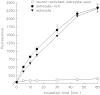

Similar articles
-
Enzymatic synthesis and characterizations of cyclic GDP-ribose. A procedure for distinguishing enzymes with ADP-ribosyl cyclase activity.J Biol Chem. 1994 Dec 2;269(48):30260-7. J Biol Chem. 1994. PMID: 7982936
-
Effect of estrogen upon cyclic ADP ribose metabolism: beta-estradiol stimulates ADP ribosyl cyclase in rat uterus.Proc Natl Acad Sci U S A. 1997 May 27;94(11):5872-6. doi: 10.1073/pnas.94.11.5872. Proc Natl Acad Sci U S A. 1997. PMID: 9159167 Free PMC article.
-
ADP-ribosyl cyclase: an enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite.Cell Regul. 1991 Mar;2(3):203-9. doi: 10.1091/mbc.2.3.203. Cell Regul. 1991. PMID: 1830494 Free PMC article.
-
Cyclic ADP-ribose and its metabolic enzymes.Biochimie. 1995;77(5):345-55. doi: 10.1016/0300-9084(96)88145-4. Biochimie. 1995. PMID: 8527488 Review.
-
ADP-ribosyl cyclase and CD38. Multi-functional enzymes in Ca+2 signaling.Adv Exp Med Biol. 1997;419:411-9. Adv Exp Med Biol. 1997. PMID: 9193683 Review.
Cited by
-
Intercellular interactions in the mammalian olfactory nerve.J Comp Neurol. 2003 Nov 10;466(2):230-9. doi: 10.1002/cne.10872. J Comp Neurol. 2003. PMID: 14528450 Free PMC article.
-
A fundamental role for the nitric oxide-G-kinase signaling pathway in mediating intercellular Ca(2+) waves in glia.J Neurosci. 2000 Mar 1;20(5):1767-79. doi: 10.1523/JNEUROSCI.20-05-01767.2000. J Neurosci. 2000. PMID: 10684878 Free PMC article.
-
Nicotinamide Adenine Dinucleotide Metabolism and Neurodegeneration.Antioxid Redox Signal. 2018 Jun 20;28(18):1652-1668. doi: 10.1089/ars.2017.7145. Epub 2017 Jun 27. Antioxid Redox Signal. 2018. PMID: 28548540 Free PMC article. Review.
-
Nicotinamide Adenine Dinucleotide (NAD+)-Dependent Signaling in Neurological Disorders.Antioxid Redox Signal. 2023 Dec;39(16-18):1150-1166. doi: 10.1089/ars.2023.0241. Epub 2023 Aug 18. Antioxid Redox Signal. 2023. PMID: 37288742 Free PMC article. Review.
References
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Clapper DL, Walseth TF, Dargie PA, Lee HC. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol triphosphate. J Biol Chem. 1987;262:9561–9568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
