Regenerative proliferation in organ cultures of the avian cochlea: identification of the initial progenitors and determination of the latency of the proliferative response
- PMID: 8757259
- PMCID: PMC6578879
- DOI: 10.1523/JNEUROSCI.16-17-05466.1996
Regenerative proliferation in organ cultures of the avian cochlea: identification of the initial progenitors and determination of the latency of the proliferative response
Abstract
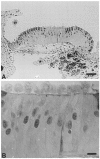
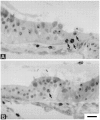
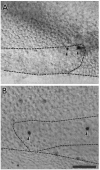
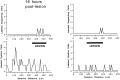
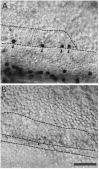
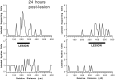
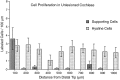
Sensory hair cells in the cochleae of birds are regenerated after the death of preexisting hair cells caused by acoustic over-stimulation or administration of ototoxic drugs. Regeneration involves renewed proliferation of cells in an epithelium that is otherwise mitotically quiescent. To determine the identity of the first cells that proliferate in response to the death of hair cells and to measure the latency of this proliferative response, we have studied hair-cell regeneration in organ culture. Cochleae from hatchling chicks were placed in culture, and hair cells were killed individually by a laser microbeam. The culture medium was then replaced with a medium that contained a labeled DNA precursor. The treated cochleae were incubated in the labeling media for different time periods before being fixed and processed for the visualization of proliferating cells. The first cells to initiate DNA replication in response to the death of hair cells were supporting cells within the cochlear sensory epithelium. All of the labeled supporting cells were located within 200 microns of the hair-cell lesions. These cells first entered S-phase approximately 16 hr after the death of hair cells. The results indicate that supporting cells are the precursors of regenerated hair cells and suggest that regenerative proliferation of supporting cells is triggered by signals that act locally within the damaged epithelium.
Figures






References
-
- Berns MW, Aist J, Edwards J, Strahs K, Girton J, McNeill P, Rattner JB, Kitzes M, Hammer-Wilson M, Liaw L-H, Siemens A, Koonce M, Peterson S, Brenner S, Burt J, Walter R, Bryant PJ, van Dyk D, Coulombe J, Cahill T, Berns GS. Laser microsurgery in cell and developmental biology. Science. 1981;213:505–513. - PubMed
-
- Brooks RF. Regulation of the fibroblast cell cycle by serum. Nature. 1976;260:248–250. - PubMed
-
- Bunting EC, Cotanche DA, Girod DA. The role of hyaline cell migration in cochlear hair cell regeneration in chick basilar papilla following severe noise damage. Assoc Res Otolaryngol Abstr. 1996;19:15.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
