Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner
- PMID: 8757265
- PMCID: PMC6578886
- DOI: 10.1523/JNEUROSCI.16-17-05536.1996
Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner
Abstract
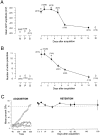
Time-dependent, learning-related changes in hippocampal excitability were evaluated by recording from rabbit CA1 pyramidal neurons in slices prepared at various times after acquisition of trace eyeblink conditioning. Increased excitability (reduced postburst afterhyperpolarizations and reduced spike-frequency adaptation) was seen as early as 1 hr after acquisition to behavioral criterion, was maximal in neurons studied 24 hr later, and returned to baseline within 7 d, whereas behavioral performance remained asymptotic for months. Neurons were held at -67 mV to equate voltage-dependent effects. No learning-related effects were observed on input resistance, action-potential amplitude or duration, or resting membrane potential. The excitability changes were learning-specific, because they were not seen in neurons from very slow learning (exhibited < 30% conditioned responses after 15 training sessions) or from pseudoconditioned control rabbits. Neurons from rabbits that displayed asymptotic behavioral performance after long-term retention testing (an additional training session 14 d after learning) were also indistinguishable from control neurons. Thus, the increased excitability of CA1 neurons was not performance- or memory-dependent. Rather, the time course of increased excitability may represent a critical window during which learning-specific alterations in postsynaptic excitability of hippocampal neurons are important for consolidation of the learned association elsewhere in the brain.
Figures

Similar articles
-
Transient changes in excitability of rabbit CA3 neurons with a time course appropriate to support memory consolidation.J Neurophysiol. 1996 Sep;76(3):1836-49. doi: 10.1152/jn.1996.76.3.1836. J Neurophysiol. 1996. PMID: 8890296
-
Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning.J Neurosci. 2000 Jul 15;20(14):5476-82. doi: 10.1523/JNEUROSCI.20-14-05476.2000. J Neurosci. 2000. PMID: 10884331 Free PMC article.
-
Aging and learning-specific changes in single-neuron activity in CA1 hippocampus during rabbit trace eyeblink conditioning.J Neurophysiol. 2001 Oct;86(4):1839-57. doi: 10.1152/jn.2001.86.4.1839. J Neurophysiol. 2001. PMID: 11600644
-
Cholinergic facilitation of trace eyeblink conditioning in aging rabbits.Life Sci. 1999;64(6-7):541-8. doi: 10.1016/s0024-3205(98)00599-2. Life Sci. 1999. PMID: 10069521 Review.
-
Age-related biophysical alterations of hippocampal pyramidal neurons: implications for learning and memory.Ageing Res Rev. 2002 Apr;1(2):181-207. doi: 10.1016/s1568-1637(01)00009-5. Ageing Res Rev. 2002. PMID: 12039438 Review.
Cited by
-
IGF-1 facilitates extinction of conditioned fear.Elife. 2021 Apr 1;10:e67267. doi: 10.7554/eLife.67267. Elife. 2021. PMID: 33792539 Free PMC article.
-
Functionally refined encoding of threat memory by distinct populations of basal forebrain cholinergic projection neurons.Res Sq [Preprint]. 2024 Feb 9:rs.3.rs-3938016. doi: 10.21203/rs.3.rs-3938016/v1. Res Sq. 2024. Update in: Elife. 2024 Feb 16;13:e86581. doi: 10.7554/eLife.86581. PMID: 38405824 Free PMC article. Updated. Preprint.
-
The role of L-type calcium channels in neuronal excitability and aging.Neurobiol Learn Mem. 2020 Sep;173:107230. doi: 10.1016/j.nlm.2020.107230. Epub 2020 May 12. Neurobiol Learn Mem. 2020. PMID: 32407963 Free PMC article. Review.
-
Dietary cholesterol degrades rabbit long term memory for discrimination learning but facilitates acquisition of discrimination reversal.Neurobiol Learn Mem. 2013 Nov;106:238-45. doi: 10.1016/j.nlm.2013.09.010. Epub 2013 Sep 25. Neurobiol Learn Mem. 2013. PMID: 24076265 Free PMC article.
-
Transcriptional and epigenetic regulation of Hebbian and non-Hebbian plasticity.Neuropharmacology. 2014 May;80:3-17. doi: 10.1016/j.neuropharm.2014.01.001. Epub 2014 Jan 10. Neuropharmacology. 2014. PMID: 24418102 Free PMC article. Review.
References
-
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. - PubMed
-
- Akase E, Alkon DL, Disterhoft JF. Hippocampal lesions impair memory of short-delay conditioned eyeblink in rabbits. Behav Neurosci. 1989;103:935–943. - PubMed
-
- Akase E, Thompson LT, Disterhoft JF. A system for quantitative analysis of associative learning. II. Real-time software for MS-DOS microcomputers. J Neurosci Methods. 1994;54:119–130. - PubMed
-
- Alkon DL. Calcium-mediated reduction of ionic currents: a biophysical memory trace. Science. 1984;226:1037–1045. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
