Chemically mediated cross-excitation in rat dorsal root ganglia
- PMID: 8764660
- PMCID: PMC6579034
- DOI: 10.1523/JNEUROSCI.16-15-04733.1996
Chemically mediated cross-excitation in rat dorsal root ganglia
Abstract
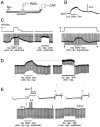
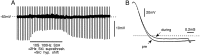
Primary afferent neurons in mammalian dorsal root ganglia (DRGs) are anatomically isolated from one another and are not synaptically interconnected. As such, they are classically thought to function as independent sensory communication elements. However, it has recently been shown that most DRG neurons are transiently depolarized when axons of neighboring neurons of the same ganglion are stimulated repetitively. Here we further characterize this functional coupling. In electrophysiological recordings made from excised rat DRGs, we found that DRG "cross-depolarization" is excitatory in that it is accompanied by an increase in the probability of spiking in response to otherwise subthreshold test pulses delivered intracellularly. Cross-depolarization contributes to this mutual cross-excitation. However, at least as important a contribution comes from a net increase in the neurons' input resistance (Rin) triggered by the stimulation of neighboring neurons. This change in Rin occurs even when cross-depolarization is absent or is balanced out. The amplitude of cross-depolaration was found to be voltage-dependent, with a reversal potential at approximately -23mV. Reversibility and the change in Rin both indicated that activity of neighboring neurons causes a membrane conductance change that is chemically mediated. Thus, far from being isolated, most DRG neurons participate in ongoing mutual interactions in which neuronal excitability is continuously modulated by afferent spike activity. This intraganglionic dialog appears to be mediated, at least in part, by an activity-dependent diffusable substance(s) released from neuronal somata and/or adjacent axons, and detected by neighboring cell somata and/or axons.
Figures






References
-
- Brown JE, Muller KJ, Murray G. Reversal potential for an electrophysiological event generated by conductance changes: mathematical analysis. Science. 1971;174:318. - PubMed
-
- Brunet PC, Jirounek P. Long-range intercellular signalling in glial cells of the peripheral nerve. NeuroReport. 1994;5:635–638. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
