Hypoglycemia prevents increase in lactic acidosis during reperfusion after temporary cerebral ischemia in rats
- PMID: 8771092
- PMCID: PMC2744691
- DOI: 10.1002/nbm.1940080406
Hypoglycemia prevents increase in lactic acidosis during reperfusion after temporary cerebral ischemia in rats
Abstract
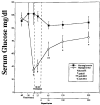
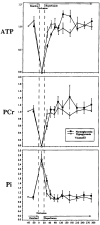
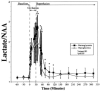
Sequential 31P and 1H MRS was used to measure cerebral phosphate metabolites, intracellular pH, and lactate in normoglycemic and hypoglycemic rats during 30 min of complete cerebral ischemia and 5.5 h of reperfusion. These results were correlated with brain levels of free fatty acids (FFAs), excitatory amino acids, cations, and water content at death. The lactate/N-acetyl aspartate ratio was not significantly different between groups before or during occlusion. During reperfusion, the ratio was higher in normoglycemic rats from 3 to 85 min (p < or = 0.05), and recovery time was faster in hypoglycemic rats (29 vs 45 min; p = 0.04), suggesting reduced lactate production and faster recovery of aerobic metabolism. During occlusion, significant but comparable decrease of intracellular pH occurred in each group. Intracellular pH was higher in hypoglycemic rats at 140 min and 260 min of reperfusion. Water content, Na and K+ concentrations, and FFA and excitatory amino acid levels were not significantly different between groups, but hypoglycemic rats had less depletion of levels of Mg2+ (p = 0.011). These results show that hypoglycemia has a limited but potentially beneficial effect on postischemic lactic acidosis.
Figures


References
-
- Ibayashi S, Fujishima M, Sadoshima S, Yoshida F, Shiokawa O, Ogata J, Omae T. Cerebral blood flow and tissue metabolism in experimental cerebral ischemia of spontaneously hypertensive rats with hyper-, normo-, and hypoglycemia. Stroke. 1986;17:261–266. - PubMed
-
- Kozuka M, Smith ML, Siesjö BK. Preischemic hyperglycemia enhances postischemic depression of cerebral metabolic rate. J. Cereb. Blood Flow Metab. 1989;9:478–490. - PubMed
-
- Plum F, Kraig RP, Pulsinelli WA. Compartmentation of acid-base in brain during complete ischemia. Neurochem. Pathol. 1988;9:139–144. - PubMed
-
- Zasslow MA, Pearl RG, Shuer LM, Steinberg GK, Lieberson RE, Larson CP., Jr Hyperglycemia decreases acute neuronal ischemic changes after middle cerebral artery occlusion in cats. Stroke. 1989;20:519–523. - PubMed
-
- Bolas NM, Rajagopalan B, Mitsumori F, Radda GK. Metabolic changes during experimental cerebral ischemia in hyperglycemic rats, observed by 31P and 1H magnetic resonance spectroscopy. Stroke. 1988;19:608–614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

