Correlation of astrocytic S100 beta expression with dystrophic neurites in amyloid plaques of Alzheimer's disease
- PMID: 8786385
- PMCID: PMC3833601
- DOI: 10.1097/00005072-199603000-00002
Correlation of astrocytic S100 beta expression with dystrophic neurites in amyloid plaques of Alzheimer's disease
Abstract
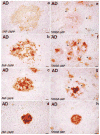
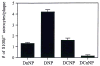
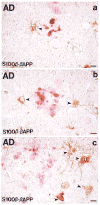
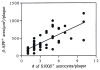
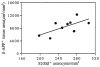
The neurite extension factor S100 beta is overexpressed by activated astrocytes associated with amyloid-containing plaques in Alzheimer's disease, and has been implicated in dystrophic neurite formation in these plaques. This predicts (a) that the appearance of S100beta- immunoreactive (S100beta+) astrocytes precedes that of dystrophic neurites in diffuse amyloid deposits and (b) that the number of these astrocytes correlates with the degree of dystrophic neurite proliferation in neuritic plaques. As a test of the first prediction, we determined the number of S100beta+ astrocytes associated with different plaque types: diffuse non-neuritic, diffuse neuritic, dense-core neuritic, and dense-core non-neuritic. Diffuse non-neuritic plaques had small numbers of associated S100beta+ astrocytes (1.3 +/- 0.1 S100beta astrocytes per plaque [mean +/- SEM]; 80% of plaques had one or more). These astrocytes were most abundant in diffuse neuritic plaques (4.2 +/- 0.2; 100%), were somewhat less numerous in dense-core neuritic plaques (1.6 +/- 0.2; 90%), and were only rarely associated with dense-core non-neuritic plaques (0.15 +/- 0.05; 12%). As a test of the second prediction, we correlated the number of S100beta+ astrocytes per plaque with the area of beta-amyloid precursor protein (beta-APP) immunoreactivity per plaque (an index of the size of the plaques' dystrophic neurite shells) and found a significant positive correlation (r = 0.74, p < 0.001). This correlation was also evident at the tissue level: the numbers of S100beta+ astrocytes per plaque-rich field correlated with the total area beta-APP immunoreactivity in these fields (r = 0.66, p < 0.05). These correlations support the idea that astrocytic activation and S100 beta overexpression are involved in the induction and maintenance of dystrophic neurites in amyloid deposits, and support the concept of a glial cytokine-mediated cascade underlying the progression of neuropathological changes in Alzheimer's disease.
Figures
Similar articles
-
Overexpression of the neuritotrophic cytokine S100beta precedes the appearance of neuritic beta-amyloid plaques in APPV717F mice.J Neurochem. 2000 Jan;74(1):295-301. doi: 10.1046/j.1471-4159.2000.0740295.x. J Neurochem. 2000. PMID: 10617132 Free PMC article.
-
[Expression of cytokine IL-1α and S100β in different types of plaques in Alzheimer's disease].Zhonghua Bing Li Xue Za Zhi. 2011 Sep;40(9):581-4. Zhonghua Bing Li Xue Za Zhi. 2011. PMID: 22177239 Chinese.
-
Interleukin-1 expression in different plaque types in Alzheimer's disease: significance in plaque evolution.J Neuropathol Exp Neurol. 1995 Mar;54(2):276-81. doi: 10.1097/00005072-199503000-00014. J Neuropathol Exp Neurol. 1995. PMID: 7876895
-
Neuritic Plaques - Gateways to Understanding Alzheimer's Disease.Mol Neurobiol. 2024 May;61(5):2808-2821. doi: 10.1007/s12035-023-03736-7. Epub 2023 Nov 8. Mol Neurobiol. 2024. PMID: 37940777 Free PMC article. Review.
-
The role of activated astrocytes and of the neurotrophic cytokine S100B in the pathogenesis of Alzheimer's disease.Neurobiol Aging. 2001 Nov-Dec;22(6):915-22. doi: 10.1016/s0197-4580(01)00293-7. Neurobiol Aging. 2001. PMID: 11754999 Review.
Cited by
-
The neuroinflammatory response in humans after traumatic brain injury.Neuropathol Appl Neurobiol. 2013 Oct;39(6):654-66. doi: 10.1111/nan.12008. Neuropathol Appl Neurobiol. 2013. PMID: 23231074 Free PMC article.
-
Impact of body mass index on neuronal fiber bundle lengths among healthy older adults.Brain Imaging Behav. 2013 Sep;7(3):300-6. doi: 10.1007/s11682-013-9230-7. Brain Imaging Behav. 2013. PMID: 23564371 Free PMC article.
-
Immunity and inflammation in neurodegenerative diseases.Am J Neurodegener Dis. 2013 Jun 21;2(2):89-107. Print 2013. Am J Neurodegener Dis. 2013. PMID: 23844334 Free PMC article.
-
Brain Atrophy, Anti-Smooth Muscle Antibody and Cognitive Impairment: An Association Study.Aging Dis. 2015 Nov 24;7(4):318-25. doi: 10.14336/AD.2015.1124. eCollection 2016 Aug. Aging Dis. 2015. PMID: 27493830 Free PMC article.
-
Overexpression of the neuritotrophic cytokine S100beta precedes the appearance of neuritic beta-amyloid plaques in APPV717F mice.J Neurochem. 2000 Jan;74(1):295-301. doi: 10.1046/j.1471-4159.2000.0740295.x. J Neurochem. 2000. PMID: 10617132 Free PMC article.
References
-
- Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42:1097–1105. - PubMed
-
- Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathological assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. - PubMed
-
- Rozemuller JM, Eikelenboom P, Stam FC, Beyreuther K, Masters CL. A4 protein in Alzheimer’s disease: Primary and secondary cellular events in extracellular amyloid deposition. J Neuropathol Exp Neurol. 1989;48:674–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

