Multiple channel types contribute to the low-voltage-activated calcium current in hippocampal CA3 pyramidal neurons
- PMID: 8795613
- PMCID: PMC6578965
- DOI: 10.1523/JNEUROSCI.16-18-05567.1996
Multiple channel types contribute to the low-voltage-activated calcium current in hippocampal CA3 pyramidal neurons
Abstract
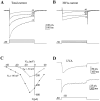
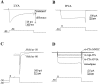
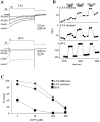
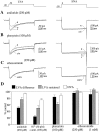
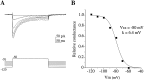
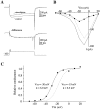
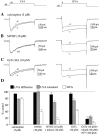
Hippocampal neurons exhibit low-voltage-activated (LVA) and high-voltage-activated (HVA) calcium currents. We characterized the LVA current by recording whole-cell Ca2+ currents from acutely isolated rat hippocampal CA3 pyramidal neurons in 2 mM Ca2+. Long depolarizing steps to -50 mV revealed two components to the LVA current: transient and sustained. The transient phase had a fast decay time constant of 59 msec. The sustained phase persisted throughout the depolarization, even for steps lasting several seconds. The transient current was inhibited by the classic T-type channel antagonists Ni2+ and amiloride. The anticonvulsant phenytoin preferentially blocked the sustained phase, but ethosuximide had no effect. Steady-state inactivation of the transient component was half-maximal at -80 mV. Nimodipine, an L-type channel antagonist, partly inhibited the sustained current. BayK-8644, an L-type channel agonist, potentiated the sustained current. Calciseptine, another L-type channel antagonist, inhibited the sustained component. omega-Conotoxin-MVIIC, a nonselective toxin for HVA channels, had no effect on either of the LVA current components. omega-Grammotoxin-SIA, another nonselective toxin, partially inhibited the sustained component. The voltage dependence of activation of the nimodipine-sensitive current could be fit with a single Boltzmann, consistent with a homogenous population of L-type channels in CA3 neurons. Half-maximal activation of the nimodipine-sensitive current occurred at -30 mV, considerably more negative than the remaining HVA current. These results suggest that in physiologic Ca2+ more than one type of Ca2+ channel contributes to the LVA current in CA3 neurons. The transient current is carried by T-type channels. The sustained current is carried, at least in part, by dihydropyridine-sensitive channels. Thus, the designation "low-voltage-activated" should not be limited to T-type channels. These findings challenge the traditional designation of L-type channels as exclusively HVA and reveal a possible role in subthreshold Ca2+ signaling.
Figures


References
-
- Armstrong CM, Matteson DR. Two distinct populations of calcium channels in a clonal line of pituitary cells. Science. 1985;227:65–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
