Modulation of excitatory synaptic transmission by adenosine released from single hippocampal pyramidal neurons
- PMID: 8795616
- PMCID: PMC6578976
- DOI: 10.1523/JNEUROSCI.16-18-05603.1996
Modulation of excitatory synaptic transmission by adenosine released from single hippocampal pyramidal neurons
Abstract
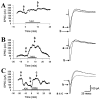
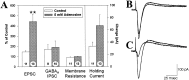
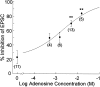
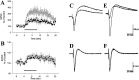
Adenosine is a potent neuromodulator in the CNS, but the mechanisms that regulate adenosine concentrations in the extracellular space remain unclear. The present study demonstrates that increasing the intracellular concentration of adenosine in a single hippocampal CA1 pyramidal neuron selectively inhibits the excitatory postsynaptic potentials in that cell. Loading neurons with high concentrations of adenosine via the whole-cell patch-clamp technique did not affect the GABAA-mediated inhibitory postsynaptic potentials, the membrane resistance, or the holding current, whereas it significantly increased the adenosine receptor-mediated depression of excitatory postsynaptic currents. The effects of adenosine could not be mimicked by an agonist at the intracellular adenosine P-site, but the effects could be antagonized by a charged adenosine receptor antagonist and by adenosine deaminase, demonstrating that the effect was mediated via adenosine acting at extracellular adenosine receptors. The effect of adenosine loading was not blocked by BaCl2 and therefore was not caused by an adenosine-activated postsynaptic potassium conductance. Adenosine loading increased the paired-pulse facilitation ratio, demonstrating that the effect was mediated by presynaptic adenosine receptors. Finally, simultaneous extracellular field recordings demonstrated that the increase in extracellular adenosine was confined to excitatory synaptic inputs to the loaded cell. These data demonstrate that elevating the intracellular concentration of adenosine in a single CA1 pyramidal neuron induces the release of adenosine into the extracellular space in such a way that it selectively inhibits the excitatory inputs to that cell, and the data support the general conclusion that adenosine is a retrograde messenger used by pyramidal neurons to regulate their excitatory input.
Figures


Similar articles
-
Ceramide-induced sustained depression of synaptic currents mediated by ionotropic glutamate receptors in the hippocampus: an essential role of postsynaptic protein phosphatases.Neuroscience. 2000;96(2):253-8. doi: 10.1016/s0306-4522(99)00582-5. Neuroscience. 2000. PMID: 10683565
-
Adenosine A1 receptor antagonist-induced facilitation of postsynaptic AMPA currents in pyramidal neurons of the rat hippocampal CA2 area.Purinergic Signal. 2023 Dec;19(4):623-632. doi: 10.1007/s11302-022-09897-9. Epub 2022 Sep 8. Purinergic Signal. 2023. PMID: 36074226 Free PMC article.
-
Modulation of AMPA receptor-mediated ion current by pituitary adenylate cyclase-activating polypeptide (PACAP) in CA1 pyramidal neurons from rat hippocampus.Hippocampus. 2009 Jan;19(1):99-109. doi: 10.1002/hipo.20488. Hippocampus. 2009. PMID: 18727050
-
Adenosine A1 receptors presynaptically modulate excitatory synaptic input onto subiculum neurons.Brain Res. 2009 Jul 14;1280:60-8. doi: 10.1016/j.brainres.2009.05.027. Epub 2009 May 18. Brain Res. 2009. PMID: 19450566 Free PMC article.
-
Hippocampal CA1 lacunosum-moleculare interneurons: comparison of effects of anoxia on excitatory and inhibitory postsynaptic currents.J Neurophysiol. 1995 Nov;74(5):2138-49. doi: 10.1152/jn.1995.74.5.2138. J Neurophysiol. 1995. PMID: 8592202
Cited by
-
Reducing Extracellular Ca2+ Induces Adenosine Release via Equilibrative Nucleoside Transporters to Provide Negative Feedback Control of Activity in the Hippocampus.Front Neural Circuits. 2017 Oct 10;11:75. doi: 10.3389/fncir.2017.00075. eCollection 2017. Front Neural Circuits. 2017. PMID: 29066955 Free PMC article.
-
The role of extracellular adenosine in regulating mossy fiber synaptic plasticity.J Neurosci. 2005 Mar 16;25(11):2832-7. doi: 10.1523/JNEUROSCI.4260-04.2005. J Neurosci. 2005. PMID: 15772343 Free PMC article.
-
A long Stokes shift red fluorescent Ca2+ indicator protein for two-photon and ratiometric imaging.Nat Commun. 2014 Oct 31;5:5262. doi: 10.1038/ncomms6262. Nat Commun. 2014. PMID: 25358432 Free PMC article.
-
Rethinking the purinergic neuron-glia connection.Proc Natl Acad Sci U S A. 2012 Apr 17;109(16):5913-4. doi: 10.1073/pnas.1203764109. Epub 2012 Apr 16. Proc Natl Acad Sci U S A. 2012. PMID: 22509008 Free PMC article. No abstract available.
-
The Purinome and the preBötzinger Complex - A Ménage of Unexplored Mechanisms That May Modulate/Shape the Hypoxic Ventilatory Response.Front Cell Neurosci. 2019 Aug 21;13:365. doi: 10.3389/fncel.2019.00365. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31496935 Free PMC article.
References
-
- Alzheimer C, Kargl L, ten Bruggencate G. Adenosinergic inhibition in hippocampus is mediated by adenosine A1 receptors very similar to those of peripheral tissues. Eur J Pharmacol. 1991;196:313–317. - PubMed
-
- Bender AS, Wu PH, Phillis JW. The characterization of [3H]adenosine uptake into rat cerebral cortical synaptosomes. J Neurochem. 1980;35:629–640. - PubMed
-
- Bender AS, Wu PH, Phillis JW. The rapid uptake and release of [3H]adenosine by rat cerebral cortical synaptosomes. J Neurochem. 1981;36:651–660. - PubMed
-
- Berne RM, Rubio R, Curnish RR. Release of adenosine from ischemic brain. Circ Res. 1974;35:262–271.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
