Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse
- PMID: 8795622
- PMCID: PMC6578977
- DOI: 10.1523/JNEUROSCI.16-18-05661.1996
Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse
Abstract
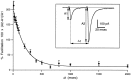
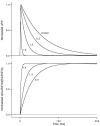
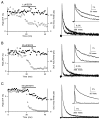
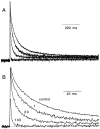
Short-term facilitation is a widely observed form of synaptic enhancement that is not well understood. Although presynaptic calcium has long been implicated in this process, its role is unclear, particularly at synapses in the mammalian brain. We tested the role of presynaptic residual free calcium ([Ca]res) in facilitation of synapses between granule cells and Purkinje cells in rat cerebellar slices. Paired-pulse facilitation of synaptic currents resulted in an approximately 2.5-fold enhancement that decayed with a time constant of approximately 200 msec, as assessed by voltage-clamp recordings. Measurements of [Ca]res using fluorescent calcium-sensitive indicators revealed that [Ca]res decayed more rapidly than did facilitation. Manipulation of [Ca]res dynamics by introducing EGTA into presynaptic terminals sped the decays of [Ca]res and facilitation in a dose-dependent manner. When [Ca]res was reduced to a brief impulse lasting several milliseconds, facilitation was still present, although reduced in amplitude and duration. Facilitation decayed with an intrinsic time constant of approximately 40 msec. These results suggest that facilitation at this synapse is produced by a calcium-driven process with a high affinity and a slow effective off-rate. A combination of [Ca]res dynamics and the properties of a calcium-driven reaction determine the time course and amplitude of facilitation.
Figures





References
-
- Augustine GJ, Neher E. Neuronal Ca2+signalling takes the local route. Curr Opin Neurobiol. 1992;2:302–307. - PubMed
-
- Bertram R, Sherman A, Stanley EF. Single-domain/bound calcium hypothesis of transmitter release and facilitation. J Neurophysiol. 1996;75:1919–1931. - PubMed
-
- Bittner GD. Synaptic plasticity at the crayfish opener neuromuscular preparation. J Neurobiol. 1989;20:386–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
