Laser ablation of Drosophila embryonic motoneurons causes ectopic innervation of target muscle fibers
- PMID: 8795627
- PMCID: PMC6578958
- DOI: 10.1523/JNEUROSCI.16-18-05715.1996
Laser ablation of Drosophila embryonic motoneurons causes ectopic innervation of target muscle fibers
Abstract
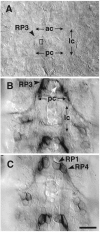
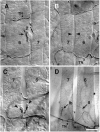
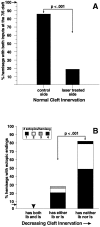
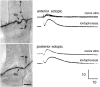
We have tested the effects of neuromuscular denervation in Drosophila by laser-ablating the RP motoneurons in intact embryos before synaptogenesis. We examined the consequences of this ablation on local synaptic connectivity in both 1st and 3rd instar larvae. We find that the partial or complete loss of native innervation correlates with the appearance of alternate inputs from neighboring motor endings and axons. These collateral inputs are found at ectopic sites on the denervated target muscle fibers. The foreign motor endings are electrophysiologically functional and are observed on the denervated muscle fibers by the 1st instar larval stage. Our data are consistent with the existence of a local signal from the target environment, which is regulated by innervation and influences synaptic connectivity. Our results show that, despite the stereotypy of Drosophila neuromuscular connections, denervation can induce local changes in connectivity in wild-type Drosophila, suggesting that mechanisms of synaptic plasticity may also be involved in normal Drosophila neuromuscular development.
Figures


References
-
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner H-R, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. - PubMed
-
- Atwood HL, Govind CK, Wu C-F. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993;24:1008–1024. - PubMed
-
- Benoit P, Changeux J-P. Consequences of tenotomy on the evolution of multi-innervation in developing rate soleus muscle. Brain Res. 1975;99:345–358. - PubMed
-
- Broadie KS, Bate M. Innervation directs receptor synthesis and localization in Drosophila embryo synaptogenesis. Nature. 1993b;361:350–353. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
