Development of an identified spinal commissural interneuron population in an amniote: neurons of the avian Hofmann nuclei
- PMID: 8795630
- PMCID: PMC6578962
- DOI: 10.1523/JNEUROSCI.16-18-05749.1996
Development of an identified spinal commissural interneuron population in an amniote: neurons of the avian Hofmann nuclei
Abstract
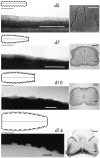
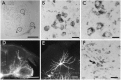
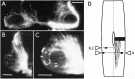
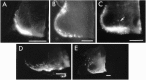
The commissural interneurons of the Hofmann nuclei (HN) of the avian spinal cord (The axonal projections of the Hofmann nuclei in the spinal cord of the late stage chicken embryo, Anat Embryol (Berl), A.L. Eide, 1996, Vol 193, pp 543-557) provide a unique opportunity to describe the development of an identified spinal commissural axon projection and its terminal collaterals in an amniote vertebrate. Here, we use the lipophilic tracer Dil to label these and other commissural projections anterogradely and retrogradely from the time the HN neurons are born. [3H]thymidine birthdating shows that the final mitoses of HN neurons occur at stages 21-24 [developmental day (d) 4]. By direct comparison, this follows the generation of motoneurons and of large, dorsally located commissural interneurons. The first HN neurons reach the ventrolateral margin of the spinal cord by d6 by a radial migration through the ventral horn. Radial migration occurs after the extension of HN axons across the midline. Thus, HN neurons are determined to be commissural interneurons before attaining their definitive locations. The HN neurons subsequently aggregate into segmentally iterated clusters at the ventrolateral margin of the spinal cord by d8. Also by d8 their logitudinal axons attain mature extent in the ventral funiculus of the contralateral side and begin to sprout collaterals. The collaterals are directed predominantly toward the medial aspect of the ventral horn at all stages, forming by d12 a dense thicket of terminals that thins out over several segments to each side of the HN of origin. The initial direction of collateral outgrowth is largely appropriate for the mature termination pattern of the HN. Terminal arbors, however, are less focused at early developmental stages than at later stages.
Figures





References
-
- Berkenblit MB, Feldman AG, Fukson OI. Wiping reflex in the frog: movement patterns, receptive fields, and blends. In: Ewert J-P, Arbib MA, editors. Visuomotor coordination: amphibians, comparisons, models and robots. Plenum; New York: 1989. pp. 615–630.
-
- Bizzi E, Mussa-Ivaldi FA, Giszter S. Computations underlying the execution of movement: a biological perspective. Science. 1991;253:287–291. - PubMed
-
- Colamarino SA, Tessier-Lavigne M. The role of the floor plate in axon guidance. Annu Rev Neurosci. 1995;18:497–529. - PubMed
-
- Dale N, Roberts A, Ottersen OP, Storm-Mathisen J. The morphology and distribution of “Kolmer-Agduhr cells,” a class of cerebrospinal-fluid-contacting neurons revealed in the frog embryo spinal cord by GABA immunocytochemistry. Proc R Soc Lond [Biol] 1987;232:193–203. - PubMed
-
- Davis B, Frank E, Johnson F, Scott S. Development of central projections of lumbosacral sensory neurons in the chick. J Comp Neurol. 1989;279:556–566. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
