Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F beta-amyloid precursor protein and Alzheimer's disease
- PMID: 8795633
- PMCID: PMC6578961
- DOI: 10.1523/JNEUROSCI.16-18-05795.1996
Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F beta-amyloid precursor protein and Alzheimer's disease
Abstract
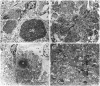
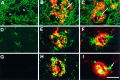
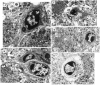
Overexpression of mutated human amyloid precursor protein (hAPP717V-->F) under control of platelet-derived growth factor promoter (PDAPP minigene) in transgenic (tg) mice results in neurodegenerative changes similar to Alzheimer's disease (AD). To clarify the pathology of these mice, we studied images derived from laser scanning confocal and electron microscopy and performed comparisons between PDAPP tg mice and AD. Similar to AD, neuritic plaques in PDAPP tg mouse contained a dense amyloid core surrounded by anti-hAPP- and antineurofilament-immunoreactive dystrophic neurites and astroglial cells. Neurons were found in close proximity to plaques in PDAPP tg mice and, to a lesser extent, in AD. In PDAPP tg mice, and occasionally in AD, neuronal processes contained fine intracellular amyloid fibrils in close proximity to the rough endoplasmic reticulum, coated vesicles, and electron-dense material. Extracellular amyloid fibrils (9-11 nm in diameter) were abundant in PDAPP tg and were strikingly similar to those observed in AD. Dystrophic neurites in plaques of PDAPP tg mouse and AD formed synapses and contained many dense multilaminar bodies and neurofilaments (10 nm). Apoptotic-like figures were present in the tg mice. No paired helical filaments have yet been observed in the heterozygote PDAPP tg mice. In summary, this study shows that PDAPP tg mice develop massive neuritic plaque formation and neuronal degeneration similar to AD. These findings show that overproduction of hAPP717V-->F in tg mice is sufficient to cause not only amyloid deposition, but also many of the complex subcellular degenerative changes associated with AD.
Figures









References
-
- Bonhomme V, Hans P, Collette J, Moonen G. Neuron-specific enolase as a marker of in vitro neuronal damage. II. Investigation of the astrocyte protective effect against kainate-induced neurotoxicity. J Neurosurg Anesthesiol. 1993;5:117–120. - PubMed
-
- Campbell G, Lieberman AR, Anderson PN, Turmaine M. Regeneration of adult rat CNS axons into peripheral nerve autografts: ultrastructural studies of the early stages of axonal sprouting and regenerative axonal growth. J Neurocytol. 1992;21:755–787. - PubMed
-
- Chartier-Harlin M-C, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, Goate A, Rossor M, Roques P, Hardy J, Mullan M. Early-onset Alzheimer’s disease caused by mutations at codon 717 of the β-amyloid precursor protein gene. Nature. 1991;353:844–846. - PubMed
-
- Clark RF, Goate AM. Molecular genetics of Alzheimer’s disease. Arch Neurol. 1993;50:1164–1172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
