Cellular organization of an antennal mechanosensory pathway in the cockroach, Periplaneta americana
- PMID: 8795635
- PMCID: PMC6578969
- DOI: 10.1523/JNEUROSCI.16-18-05830.1996
Cellular organization of an antennal mechanosensory pathway in the cockroach, Periplaneta americana
Abstract
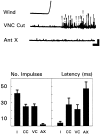
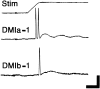
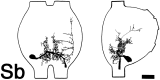
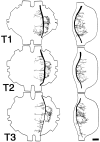
Escape responses of cockroaches, Periplaneta americana, can be triggered by wind and mediated by a group of "giant interneurons" that ascend from cercal mechanoreceptors to motor centers. Recently it has been observed that escape also can be triggered by tactile stimulation of the antennae, and it is then independent of the giant interneurons. Here we identify a descending antennal mechanosensory pathway that may account for escape. Cobalt backfills demonstrated that a limited number of cells in the head ganglia have axons that project through all three thoracic ganglia. Comparison with known wind-sensory pathways indicated that wind is not a reliable stimulus for activating descending antennal pathways. However, direct touch stimulation of an antenna reliably evoked short-latency responses in cells with axons in the cervical connectives. Intracellular recording and dye injection revealed members of this pathway, referred to as descending mechanosensory interneurons (DMIs). The two axons of largest diameter in the cervical connectives were found to belong to DMIs, and these large-caliber interneurons were studied in detail. One had a soma in the supraesophageal ganglion, and the other in the subesophageal ganglion. Both had extensive neuritic arborizations at the same level as the soma and axonal arbors in all three thoracic ganglia. Each of these DMIs exhibited short-latency responses to small antennal movements, demonstrated a degree of directional sensitivity, and rapidly conducted impulses to thoracic levels. These cells have properties suggesting that they play a role in a short-latency behavior such as touch-evoked escape.
Figures







References
-
- Arbas EA. Control of hindlimb posture by wind-sensitive hairs and antennae during locust flight. J Comp Physiol [A] 1986;159:849–857. - PubMed
-
- Bacon JP, Altman JS. A silver intensification method for cobalt-filled neurons in whole-mount preparations. Brain Res. 1977;138:359–363. - PubMed
-
- Bacon J, Möhl B. The tritocerebral commissure giant (TCG) wind-sensitive interneurone in the locust. I. Its activity in straight flight. J Comp Physiol [A] 1983;150:439–452.
-
- Bacon JP, Strausfeld NJ. The dipteran giant fibre pathway: neurons and signals. J Comp Physiol [A] 1986;158:529–548.
-
- Bicker G, Pearson KG. Initiation of flight by an identified wind-sensitive neurone (TCG) in the locust. J Exp Biol. 1983;104:289–293.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
