Hair cell differentiation in chick cochlear epithelium after aminoglycoside toxicity: in vivo and in vitro observations
- PMID: 8815898
- PMCID: PMC6579194
- DOI: 10.1523/JNEUROSCI.16-19-06157.1996
Hair cell differentiation in chick cochlear epithelium after aminoglycoside toxicity: in vivo and in vitro observations
Abstract
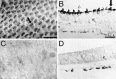
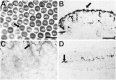
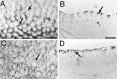
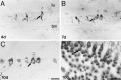
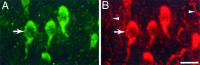
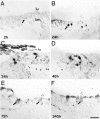
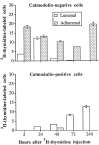
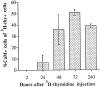
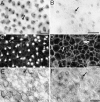
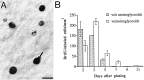
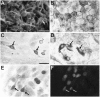
Inner ear epithelia of mature birds regenerate hair cells after ototoxic or acoustic insult. The lack of markers that selectively label cells in regenerating epithelia and of culture systems composed primarily of progenitor cells has hampered the identification of cellular and molecular interactions that regulate hair cell regeneration. In control basilar papillae, we identified two markers that selectively label hair cells (calmodulin and TUJ1 beta tubulin antibodies) and one marker unique for support cells (cytokeratin antibodies). Examination of regenerating epithelia demonstrated that calmodulin and beta tubulin are also expressed in early differentiating hair cells, and cytokeratins are retained in proliferative support cells. Enzymatic and mechanical methods were used to isolate sensory epithelia from mature chick basilar papillae, and epithelia were cultured in different conditions. In control cultures, hair cells are morphologically stable for up to 6 d, because calmodulin immunoreactivity and phalloidin labeling of filamentous actin are retained. The addition of an ototoxic antibiotic to cultures, however, causes complete hair cell loss by 2 d in vitro and generates cultures composed of calmodulin-negative, cytokeratin-positive support cells. These cells are highly proliferative for the first 2-7 d after plating, but stop dividing by 9 d. Calmodulin- or TUJ1-positive cells reemerge in cultures treated with antibiotic for 5 d and maintained for an additional 5 d without antibiotic. A subset of calmodulin-positive cells was also labeled with BrdU when it was continuously present in cultures, suggesting that some cells generated in culture begin to differentiate into hair cells.
Figures



References
-
- Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci Lett. 1996;205:17–20. - PubMed
-
- Anniko M, Thornell LE, Ramaekers FC, Stigbrand T. Cytokeratin diversity in epithelia of the human inner ear. Acta Otolaryngol (Stockh) 1989;108:385–396. - PubMed
-
- Anniko M, Arnold W, Thornell LE, Virtanen I, Ramaekers FC, Pfaltz CR. Regional variations in the expression of cytokeratin proteins in the adult human cochlea. Eur Arch Otolaryngol. 1990;247:182–188. - PubMed
-
- Arnold W, Anniko M. Cytoskeletal network of intermediate filament proteins in the adult human vestibular labyrinth. Acta Otolaryngol [Suppl] (Stockh) 1990;470:40–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
