Aging changes in voltage-gated calcium currents in hippocampal CA1 neurons
- PMID: 8815908
- PMCID: PMC6579167
- DOI: 10.1523/JNEUROSCI.16-19-06286.1996
Aging changes in voltage-gated calcium currents in hippocampal CA1 neurons
Abstract
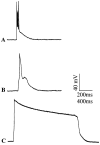
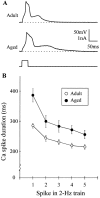
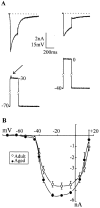
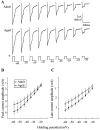
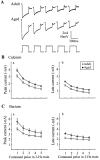
Previous current-clamp studies in rat hippocampal slice CA1 neurons have found aging-related increases in long-lasting calcium (Ca)-dependent and Ca-mediated potentials. These changes could reflect an increase in Ca influx through voltage-gated Ca channels but also could reflect a change in potassium currents. Moreover, if altered Ca influx is involved, it is nuclear whether it arises from generally increased Ca channel activity, lower threshold, or reduced inactivation. To analyze the basis for altered Ca potentials, whole-cell voltage-clamp studies of CA1 hippocampal neurons were performed in nondissociated hippocampal slices of adult (3- to 5-month-old) and aged (25- to 26-month-old) rats. An aging-related increase was found in high-threshold Ca and barium (Ba) currents, particularly in the less variable, slowly inactivating (late) current at the end of a depolarization step. Input resistance of neurons did not differ between age groups. In steady-state inactivation and repetitive-pulse protocols, inactivation of Ca and Ba currents was not reduced and, in some cases, was slightly greater in aged neurons, apparently because of larger inward current. The current blocked by nimodipine was greater in aged neurons, indicating that some of the aging increase was in L-type currents. These results indicate that whole-cell Ca currents are increased with aging in CA1 neurons, apparently attributable to greater channel activity rather than to reduced inactivation. The elevated Ca influx seems likely to play a role in impaired function and enhanced susceptibility to neurotoxic influences.
Figures
Similar articles
-
High-voltage-activated calcium currents in basal forebrain neurons during aging.J Neurophysiol. 1996 Jul;76(1):158-74. doi: 10.1152/jn.1996.76.1.158. J Neurophysiol. 1996. PMID: 8836216
-
Aging-related increase in hippocampal calcium channels.Life Sci. 1996;59(5-6):399-404. doi: 10.1016/0024-3205(96)00318-9. Life Sci. 1996. PMID: 8761327 Review.
-
Effects of extracellular pH on voltage-gated Na+, K+ and Ca2+ currents in isolated rat CA1 neurons.J Physiol. 1996 Jun 15;493 ( Pt 3)(Pt 3):719-32. doi: 10.1113/jphysiol.1996.sp021417. J Physiol. 1996. PMID: 8799894 Free PMC article.
-
Dendritic voltage-gated ion channels regulate the action potential firing mode of hippocampal CA1 pyramidal neurons.J Neurophysiol. 1999 Oct;82(4):1895-901. doi: 10.1152/jn.1999.82.4.1895. J Neurophysiol. 1999. PMID: 10515978
-
Functional aspects of calcium-channel modulation.Clin Neuropharmacol. 1993;16 Suppl 1:S12-24. doi: 10.1097/00002826-199316001-00003. Clin Neuropharmacol. 1993. PMID: 8390916 Review.
Cited by
-
Molecular and cellular aspects of age-related cognitive decline and Alzheimer's disease.Behav Brain Res. 2017 Mar 30;322(Pt B):191-205. doi: 10.1016/j.bbr.2016.05.008. Epub 2016 May 7. Behav Brain Res. 2017. PMID: 27163751 Free PMC article. Review.
-
Experiments in macaque monkeys provide critical insights into age-associated changes in cognitive and sensory function.Proc Natl Acad Sci U S A. 2019 Dec 26;116(52):26247-26254. doi: 10.1073/pnas.1902279116. Epub 2019 Dec 23. Proc Natl Acad Sci U S A. 2019. PMID: 31871147 Free PMC article.
-
Increased calcium channel in the lamina propria of aging rat.Aging (Albany NY). 2019 Oct 31;11(20):8810-8824. doi: 10.18632/aging.102284. Epub 2019 Oct 31. Aging (Albany NY). 2019. PMID: 31682233 Free PMC article.
-
Aging is associated with elevated intracellular calcium levels and altered calcium homeostatic mechanisms in hippocampal neurons.Neurosci Lett. 2007 May 11;418(1):77-81. doi: 10.1016/j.neulet.2007.03.005. Epub 2007 Mar 12. Neurosci Lett. 2007. PMID: 17374449 Free PMC article.
-
TRPC3 channels critically regulate hippocampal excitability and contextual fear memory.Behav Brain Res. 2015 Mar 15;281:69-77. doi: 10.1016/j.bbr.2014.12.018. Epub 2014 Dec 13. Behav Brain Res. 2015. PMID: 25513972 Free PMC article.
References
-
- Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994;17:8–13. - PubMed
-
- Barnes CA, McNaughton BL, O’Keffe J. Loss of place specificity in hippocampal complex spike cells of senescent rat. Neurobiol Aging. 1983;4:113–119. - PubMed
-
- Bean BP. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
