Neural delays shape selectivity to interaural intensity differences in the lateral superior olive
- PMID: 8815932
- PMCID: PMC6578907
- DOI: 10.1523/JNEUROSCI.16-20-06554.1996
Neural delays shape selectivity to interaural intensity differences in the lateral superior olive
Abstract
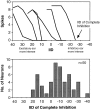
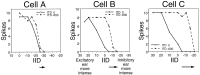
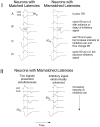
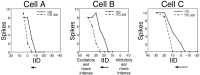
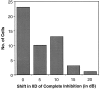
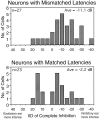
Neurons in the lateral superior olive (LSO) respond selectively to interaural intensity differences (IIDs), one of the chief cues used to localize sounds in space. LSO cells are innervated in a characteristic pattern: they receive an excitatory input from the ipsilateral ear and an inhibitory input from the contralateral ear. Consistent with this pattern, LSO cells generally are excited by sounds that are more intense at the ipsilateral ear and inhibited by sounds that are more intense at the contralateral ear. Despite their relatively homogeneous pattern of innervation, IID selectivity varies substantially from cell to cell, such that selectivities are distributed over the range of IIDs that would be encountered in nature. For some time, researchers have speculated that the relative timing of the excitatory and inhibitory inputs to an LSO cell might shape IID selectivity. To test this hypothesis, we recorded from 50 LSO cells in the free-tailed bat while presenting stimuli that varied in interaural intensity and in interaural time of arrival. The results suggest that, for more than half of the cells, the latency of inhibition was several hundred microseconds longer than the latency of excitation. Increasing the intensity to the inhibitory ear shortened the latency of inhibition and brought the timing of the inputs from the two ears into register. Thus, a neural delay of the inhibition helped to define the IID selectivity of these cells, accounting for a significant part of the variation in selectivity among LSO cells.
Figures





References
-
- Blum JJ, Reed MC. Further studies of a model for azimuthal encoding: lateral superior olive neuron–response curves and developmental processes. J Acoust Soc Am. 1991;90:1968–1978. - PubMed
-
- Boudreau JC, Tsuchitani C. Binaural interaction in the cat superior olive S-segment. J Neurophysiol. 1968;31:442–454. - PubMed
-
- Caird D, Klinke R. Processing of binaural stimuli by cat superior olivary complex neurons. Exp Brain Res. 1983;52:385–399. - PubMed
-
- Covey E, Vater M, Casseday JH. Binaural properties of single units in the superior olivary complex of the mustached bat. J Neurophysiol. 1991;66:1080–1094. - PubMed
-
- Erulkar SD. Comparative aspects of spatial localization of sounds. Physiol Rev. 1972;52:237–360. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
