Localization of rhythmogenic networks responsible for spontaneous bursts induced by strychnine and bicuculline in the rat isolated spinal cord
- PMID: 8824342
- PMCID: PMC6579249
- DOI: 10.1523/JNEUROSCI.16-21-07063.1996
Localization of rhythmogenic networks responsible for spontaneous bursts induced by strychnine and bicuculline in the rat isolated spinal cord
Abstract
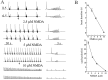
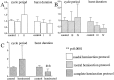
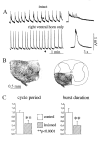
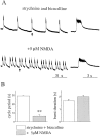
Spontaneous rhythmic bursting induced by coapplication of strychnine (1 microM) and bicuculline (20 microM) was observed with electrophysiological recording from pairs of lumbar ventral roots (usually L5) in an isolated preparation of the neonatal rat spinal cord. Bursting was insensitive to exogenously applied GABA or glycine, confirming that it was attributable to block of glycine and GABAA receptor-mediated inhibition. NMDA accelerated bursting in a dose-dependent manner. Complete coronal spinal transection at L3 or L6 level did not block bursting recorded from L5 or L2 roots, respectively. Gradual cutting of the cord along the midline through a sagittal plane preserved bursting activity in both disconnected sides but led to loss of synchronicity. Once the spinal cord was fully separated into left and right halves, regular bursting persisted on each side with no phase-coupling between the two preparations. Section along a frontal plane to remove dorsal horns and much of the central canal area did not affect burst frequency or left-to-right synchronicity, whereas it reduced burst duration. A quadrant preparation containing mainly a single ventral horn displayed enhanced burst frequency while bursts became very short events. Bath application of 5-hydroxytryptamine (30 microM) or NMDA (5 microM) increased burst frequency and decreased burst duration in all types of preparation except the isolated quadrants, in which brief bursts were accelerated but not shortened by these chemical agents. These results suggest that bursting induced by strychnine and bicuculline apparently relied on distinct mechanisms for burst triggering and intraburst structure. The first required a relatively smaller neuronal network that was confined to a ventral quadrant. Intraburst structure was dependent on a larger circuitry comprising either both ventral horns or one side of the spinal cord including more than two segments.
Figures





References
-
- Ballerini L, Bracci E, Nistri A. Desensitization of AMPA receptors limits the amplitude of EPSPs and the excitability of motoneurons of the rat isolated spinal cord. Eur J Neurosci. 1995;7:1229–1234. - PubMed
-
- Bracci E, Ballerini L, Nistri A. Spontaneous rhythmic bursts induced by pharmacological block of inhibition in lumbar motoneurons of the neonatal rat spinal cord. J Neurophysiol. 1996;75:640–647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
