In vitro translation of the full-length RNA transcript of figwort mosaic virus (Caulimovirus)
- PMID: 8882638
- PMCID: PMC6138018
In vitro translation of the full-length RNA transcript of figwort mosaic virus (Caulimovirus)
Abstract
The circular DNA genome of FMV consists of seven tandemly arranged genes placed successively on a full-length RNA transcript that spans the entire circular viral genome. This transcript is a tentative mRNA for at least five of the six major conserved genes of this virus (genes I-V) that are positioned on this transcript. The sixth major gene (gene VI) is expressed as a separate monocistronic transcript. A long 5'-nontranslated leader (598 nucleotides), a small nonconserved gene (VII), and a short intergenic region (57 nucleotides) precede the five major conserved genes (I through V) on the full-length transcript. A reporter gene (CAT), as a separate cistron or fused in-frame, to viral cistrons in various downstream positions in cloned versions of the viral genome was used in a transcription vector to generate artificial full-length transcripts of FMV. When these mRNAs were translated in vitro (rabbit reticulocyte lysate system), the reporter gene was translated efficiently in all positions. Translation of internal native viral gene positioned on the full-length transcript of FMV was also determined (the gene VI product). These observations suggest that the full-length FMV transcript functions as a polycistronic mRNA in plants. Results are best explained on the basis of translational coupling/relay race model.
Figures

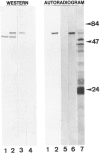



Similar articles
-
Gene VI of figwort mosaic virus (caulimovirus group) functions in posttranscriptional expression of genes on the full-length RNA transcript.Proc Natl Acad Sci U S A. 1989 Dec;86(23):9203-7. doi: 10.1073/pnas.86.23.9203. Proc Natl Acad Sci U S A. 1989. PMID: 2594762 Free PMC article.
-
Requirement of gene VII in cis for the expression of downstream genes on the major transcript of figwort mosaic virus.Virology. 1991 Dec;185(2):867-71. doi: 10.1016/0042-6822(91)90561-o. Virology. 1991. PMID: 1962457
-
The full-length transcript of a caulimovirus is a polycistronic mRNA whose genes are trans activated by the product of gene VI.J Virol. 1992 May;66(5):3131-9. doi: 10.1128/JVI.66.5.3131-3139.1992. J Virol. 1992. PMID: 1560539 Free PMC article.
-
Efficient translation of distal cistrons of a polycistronic mRNA of a plant pararetrovirus requires a compatible interaction between the mRNA 3' end and the proteinaceous trans-activator.Virology. 1996 Oct 15;224(2):564-7. doi: 10.1006/viro.1996.0565. Virology. 1996. PMID: 8874519
-
Pararetroviruses and retroviruses: a comparative review of viral structure and gene expression strategies.Adv Virus Res. 1994;44:1-67. doi: 10.1016/s0065-3527(08)60327-9. Adv Virus Res. 1994. PMID: 7817872 Review. No abstract available.
Cited by
-
Cloning and expression of 1-aminocyclopropane-1-carboxylate synthase cDNA from rosa (Rosa x hybrida).Plant Cell Rep. 2004 Jan;22(6):422-9. doi: 10.1007/s00299-003-0721-7. Epub 2003 Oct 25. Plant Cell Rep. 2004. PMID: 14579075
References
-
- Al Ani R.; Pfeiffer P.; Whitechurch O.; Lesot A.; Lebeurier G.; Hirth L. Ann. Virol. (Inst. Pasteur) 131E:33–53; 1980.
-
- Bonneville J. M.; Sanfacon H.; Fütterer J.; Hohn T. Cell 59:1135–1143; 1989. - PubMed
-
- Chomczynski P.; Sacchi N. Anal. Biochem. 162:156–159; 1987. - PubMed
-
- Covey S. N.; Hull R. Virology 111:463–474; 1981. - PubMed
-
- Davis L. G.; Mark D.; Battey J. F. In: Basic methods in molecular biology. New York: Elsevier Science, Inc.; 1986:143–146.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
