Development of pilus organelle subassemblies in vitro depends on chaperone uncapping of a beta zipper
- PMID: 8917515
- PMCID: PMC24016
- DOI: 10.1073/pnas.93.23.12890
Development of pilus organelle subassemblies in vitro depends on chaperone uncapping of a beta zipper
Abstract
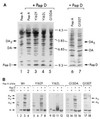
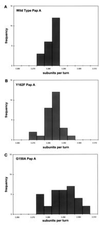
The major subassemblies of virulence-associated P pili, the pilus rod (comprised of PapA) and tip fibrillum (comprised of PapE), were reconstituted from purified chaperone-subunit complexes in vitro. Subunits are held in assembly-competent conformations in chaperone-subunit complexes prior to their assembly into mature pili. The PapD chaperone binds, in part, to a conserved motif present at the C terminus of the subunits via a beta zippering interaction. Amino acid residues in this conserved motif were also found to be essential for subunit-subunit interactions necessary for the formation of pili, thus revealing a molecular mechanism whereby the PapD chaperone may prevent premature subunit-subunit interactions in the periplasm. Uncapping of the chaperone-protected C terminus of PapA and PapE was mimicked in vitro by freeze-thaw techniques and resulted in the formation of pilus rods and tip fibrillae, respectively. A mutation in the leading edge of the beta zipper of PapA produces pilus rods with an altered helical symmetry and azimuthal disorder. This change in the number of subunits per turn of the helix most likely reflects involvement of the leading edge of the beta zipper in forming a right-handed helical cylinder. Organelle development is a fundamental process in all living cells, and these studies shed new light on how immunoglobulin-like chaperones govern the formation of virulence-associated organelles in pathogenic bacteria.
Figures


References
-
- Hultgren S J, Abraham S N, Caparon M G, Falk P, St. Geme J W, III, Normark S. Cell. 1993;73:887–901. - PubMed
-
- Hultgren S J, Normark S, Abraham S N. Annu Rev Microbiol. 1991;45:383–415. - PubMed
-
- Kuehn M J, Heuser J, Normark S, Hultgren S J. Nature (London) 1992;356:252–255. - PubMed
-
- Gong M, Makowski L. J Mol Biol. 1992;228:735–742. - PubMed
-
- Bullitt E, Makowski L. Nature (London) 1995;373:164–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

