Activation of mouse sperm T-type Ca2+ channels by adhesion to the egg zona pellucida
- PMID: 8917534
- PMCID: PMC24036
- DOI: 10.1073/pnas.93.23.13004
Activation of mouse sperm T-type Ca2+ channels by adhesion to the egg zona pellucida
Abstract
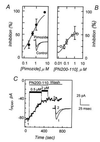
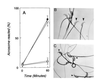
The sperm acrosome reaction is a Ca(2+)-dependent exocytotic event that is triggered by adhesion to the mammalian egg's zona pellucida. Previous studies using ion-selective fluorescent probes suggested a role of voltage-sensitive Ca2+ channels in acrosome reactions. Here, wholecell patch clamp techniques are used to demonstrate the expression of functional T-type Ca2+ channels during mouse spermatogenesis. The germ cell T current is inhibited by antagonists of T-type channels (pimozide and amiloride) as well as by antagonists whose major site of action is the somatic cell L-type Ca2+ channel (1,4-dihydropyridines, arylalkylamines, benzothiazapines), as has also been reported for certain somatic cell T currents. In sperm, inhibition of T channels during gamete interaction inhibits zona pellucida-dependent Ca2+ elevations, as demonstrated by ion-selective fluorescent probes, and also inhibits acrosome reactions. These studies directly link sperm T-type Ca2+ channels to fertilization. In addition, the kinetics of channel inhibition by 1,4-dihydropyridines suggests a mechanism for the reported contraceptive effects of those compounds in human males.
Figures
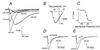

References
-
- Yanagimachi R. In: The Physiology of Reproduction. Knobil E, Neill J D, editors. New York: Raven; 1994. pp. 189–317.
-
- Babcock D F, Pfeiffer D R. J Biol Chem. 1987;262:15041–15047. - PubMed
-
- Florman H M, Corron M E, Kim T D-H, Babcock D F. Dev Biol. 1992;152:304–314. - PubMed
-
- Florman H M. Dev Biol. 1994;165:152–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

