Evidence that the 42- and 40-amino acid forms of amyloid beta protein are generated from the beta-amyloid precursor protein by different protease activities
- PMID: 8917563
- PMCID: PMC24065
- DOI: 10.1073/pnas.93.23.13170
Evidence that the 42- and 40-amino acid forms of amyloid beta protein are generated from the beta-amyloid precursor protein by different protease activities
Abstract
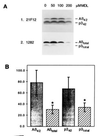
Cerebral deposition of the amyloid beta protein (A beta) is an early and invariant feature of Alzheimer disease (AD). Whereas the 40-amino acid form of A beta (A beta 40) accounts for approximately 90% of all A beta normally released from cells, it appears to contribute only to later phases of the pathology. In contrast, the longer more amyloidogenic 42-residue form (A beta 42), accounting for only approximately 10% of secreted A beta, is deposited in the earliest phase of AD and remains the major constituent of most amyloid plaques throughout the disease. Moreover, its levels have been shown to be increased in all known forms of early-onset familial AD. Thus, inhibition of A beta 42 production is a prime therapeutic goal. The same protease, gamma-secretase, is assumed to generate the C termini of both A beta 40 and A beta 42. Herein, we analyze the effect of the compound MDL 28170, previously suggested to inhibit gamma-secretase, on beta-amyloid precursor protein processing. By immunoprecipitating conditioned medium of different cell lines with various A beta 40- and A beta 42-specific antibodies, we demonstrate a much stronger inhibition of the gamma-secretase cleavage at residue 40 than of that at residue 42. These data suggest that different proteases generate the A beta 40 and A beta 42 C termini. Further, they raise the possibility of identifying compounds that do not interfere with general beta-amyloid precursor protein metabolism, including A beta 40 production, but specifically block the generation of the pathogenic A beta 42 peptide.
Figures



References
-
- Joachim C L, Duffy L K, Morris J H, Selkoe D J. Brain Res. 1988;474:100–111. - PubMed
-
- Castano E M, Frangione B. Lab Invest. 1988;58:122–132. - PubMed
-
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina H, Ihara Y. Neuron. 1994;13:45–53. - PubMed
-
- Iwatsubo T, Mann D M, Odaka A, Suzuki N, Ihara Y. Ann Neurol. 1995;37:294–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

