Intrinsic injury signals enhance growth, survival, and excitability of Aplysia neurons
- PMID: 8922402
- PMCID: PMC6579080
- DOI: 10.1523/JNEUROSCI.16-23-07469.1996
Intrinsic injury signals enhance growth, survival, and excitability of Aplysia neurons
Abstract
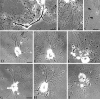
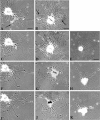
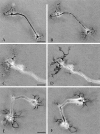
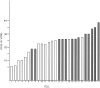
Neurons undergo extensive changes in growth and electrophysiological properties in response to axon injury. Efforts to understand the molecular mechanisms that initiate these changes have focused almost exclusively on the role of extrinsic signals, primarily neurotrophic factors released from target and glial cells. The objective of the present investigation was to determine whether the response to axonal injury also involves intrinsic axoplasmic signals. Aplysia neurons were removed from their ganglia and placed in vitro on a substratum permissive for growth, but in the absence of glia and soluble growth factors. Under these conditions, neurites emerged and grew for approximately 4 d. Once growth had ceased, the neurites were transected. In all, 46 of 50 cells regenerated, either by resorbing the remaining neurites and elaborating a new neuritic arbor or by merely replacing the neurites that had been severed. Cut cells also exhibited enhanced excitability and, paradoxically, prolonged survival, when compared with uninjured neurons. These findings indicate that axons contain intrinsic molecular signals that are directly activated by injury to trigger changes underlying regeneration and compensatory plasticity.
Figures

References
-
- Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Neuron. 1994;76:1099–1114. - PubMed
-
- Alberini CM, Ghirardi M, Huang Y-Y, Nguyen PV, Kandel ER. A molecular switch for the consolidation of long-term memory: cAMP-inducible gene expression. Ann NY Acad Sci. 1995;256:261–286. - PubMed
-
- Ambron RT, Walters ET. Priming events and retrograde injury signals: a new perspective on the cellular and molecular biology of nerve regeneration. Mol Neurobiol. 1996;13:61–79. - PubMed
-
- Ambron RT, Goldman JE, Schwartz JH. Effect of inhibiting protein synthesis on axonal transport of membrane glycoproteins in an identified neuron of Aplysia. Brain Res. 1975;94:307–323. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
