Cellular mechanisms of the augmenting response: short-term plasticity in a thalamocortical pathway
- PMID: 8922430
- PMCID: PMC6579081
- DOI: 10.1523/JNEUROSCI.16-23-07742.1996
Cellular mechanisms of the augmenting response: short-term plasticity in a thalamocortical pathway
Abstract
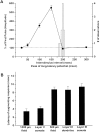
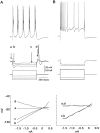
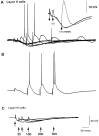
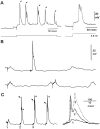
Some thalamocortical pathways display an "augmenting response" when stimuli are delivered at frequencies between 7 and 14 Hz. Cortical responses to the first three stimuli of a series increase progressively in amplitude and are relatively stable thereafter. We have investigated the cellular mechanisms of the augmenting response using extracellular and intracellular recordings in vivo and in slices of the sensorimotor neocortex of the rat. Single stimuli to the ventrolateral (VL) nucleus of the thalamus generate EPSPs followed by feedforward IPSPs that hyperpolarize cells in layer V. A long-latency depolarization interrupts the IPSP with a peak at approximately 200 msec. A second VL stimulus delivered during the hyperpolarization and before the peak of the long-latency depolarization yields an augmenting response. The shortest latency for augmenting responses occurs in cells of layer V, and they appear in dendrites and somata recorded in upper layers approximately 5 msec later. Recordings in vitro show that some layer V cells have hyperpolarization-activated and deinactivated conductances that may serve to increase their excitability after IPSPs. Also in vitro, cells from layer V, but not from layer III, generated augmenting responses at the same stimulation frequencies that were effective in vivo. Control experiments indicated that neither paired-pulse depression of IPSPs nor presynaptically mediated facilitation can account for the augmenting response. Active dendritic conductances contribute to the spread of augmenting responses into upper layers by way of back-propagating fast spikes, which attenuate with repetition, and long-lasting spikes, which enhance in parallel with the augmenting response. In conclusion, we propose that the initiation of augmenting responses depends on an interaction between inhibition, intrinsic membrane properties, and synaptic interconnections of layer V pyramidal neurons.
Figures





References
-
- Addae JI, Stone TW. Involvement of N-methyl-d-aspartate receptors in the augmenting response in rat neocortex. Neurosci Lett. 1987;78:323–327. - PubMed
-
- Agmon R, Connors BW. Repetitive burst-firing neurons in the deep layers of mouse somatosensory cortex. Neurosci Lett. 1989;99:137–141. - PubMed
-
- Amitai Y, Friedman A, Connors BW, Gutnick MJ. Regenerative activity in apical dendrites of pyramidal cells in neocortex. Cereb Cortex. 1993;3:26–38. - PubMed
-
- Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res. 1991;88:501–520. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
