Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus
- PMID: 8929424
- PMCID: PMC6578928
- DOI: 10.1523/JNEUROSCI.16-22-07151.1996
Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus
Abstract
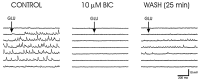
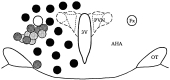
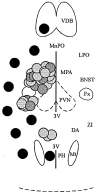
Local inhibitory synaptic inputs to neurons of the rat hypothalamic paraventricular nucleus (PVN) were studied by using glutamate microstimulation and conventional intracellular and whole-cell patch-clamp recording in coronal, horizontal, and parasagittal slices of rat hypothalamus. PVN cells were classified as magnocellular or parvocellular neurons on the basis of electrophysiological and post hoc immunohistochemical analyses; GABA-producing neurons were localized with in situ hybridization. Glutamate microstimulation of different sites around the PVN evoked volleys of postsynaptic potentials in 43% of the PVN neurons tested. Some responses to stimulation at each site were blocked by bicuculline, suggesting that they were mediated by the activation of presynaptic GABA neurons. In the coronal plane, presynaptic inhibitory sites were located lateral to the PVN and ventral to the fornix, corresponding to the lateral hypothalamic area and the posterior bed nucleus of the stria terminalis (BNST). In the horizontal plane, presynaptic inhibitory sites were found rostral, lateral, and caudal to the nucleus, corresponding to parts of the anterior hypothalamic area, the posterior BNST, the medial preoptic area, and the dorsomedial hypothalamus. In the parasagittal plane, presynaptic inhibitory neurons were revealed at sites rostral and caudal to the nucleus, corresponding to the medial preoptic area and the dorsomedial hypothalamus, and in a site dorsal to the optic chiasm that included the suprachiasmatic nucleus. These presynaptic sites each contained GABA-producing neurons based on in situ hybridization with a glutamic acid decarboxylase riboprobe and together formed a three-dimensional ring around the PVN. Unexpectedly, both magnocellular and parvocellular neurons received inhibitory synaptic inputs from common sites.
Figures






References
-
- Altstein M, Whitnall MH, House S, Key S, Gainer H. An immunochemical analysis of oxytocin and vasopressin prohormone processing in vivo. Peptides. 1988;9:87–105. - PubMed
-
- Arluison M, Brochier G, Vankova M, Leviel V, Villalobos J, Tramu G. Demonstration of peptidergic afferents to the bed nucleus of the stria terminalis using local injections of colchicine. A combined immunohistochemical and retrograde tracing study. Brain Res Bull. 1994;34:319–337. - PubMed
-
- Boudaba C, Poulain DA. Further evidence that the septum is not part of the main pathway of the milk ejection in the rat. J Neuroendocrinol. 1991;3:199–204. - PubMed
-
- Boudaba C, Szabó K, Tasker JG. Physiological mapping of local inhibitory neurons which project to the paraventricular nucleus in rat hypothalamic slices. Soc Neurosci Abstr. 1995;21:1665.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
