A role in migration for the alpha V beta 1 integrin expressed on oligodendrocyte precursors
- PMID: 8929432
- PMCID: PMC6578950
- DOI: 10.1523/JNEUROSCI.16-22-07240.1996
A role in migration for the alpha V beta 1 integrin expressed on oligodendrocyte precursors
Abstract
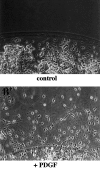
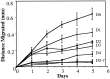
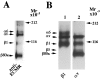
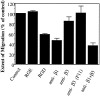
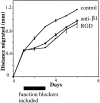
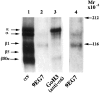
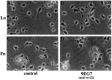
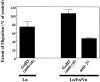
Myelination of the CNS requires the migration of oligodendrocyte precursors throughout the CNS from restricted regions within the ventricular and subventricular zones. In light of the significant effects of cell-extracellular matrix (ECM) interactions on cell migration in other developing systems, we have analyzed the role of integrins in oligodendrocyte precursor migration. We have shown previously that oligodendrocyte precursors in vitro express a limited repertoire of integrins, including alpha 6 beta 1, alpha v beta 3, and that differentiation is associated with downregulation of alpha v beta 1 and upregulation of alpha v beta 5. Using a migration assay based on the movement of cells away from an agarose drop containing a high-density cell suspension, we find that RGD peptides (that block alpha v but not alpha 6 integrins) and anti-beta 1 antibodies block migration on an astrocyte-derived ECM, whereas anti-beta 3 antibodies have little effect. These results suggest that alpha v beta 1 but not alpha 6 beta 1 plays a role in oligodendrocyte precursor migration, and this is confirmed by the use of blocking monoclonal antibodies that distinguish these two integrins. In keeping with the results of others, we find that differentiated oligodendrocytes lose migratory potential and that the timing of this loss correlates with downregulation of alpha v beta 1. Taken together with the work of others showing that ECM ligands for alpha v beta 1 are expressed within the CNS, we propose that this integrin plays a significant role in the migration of oligodendrocyte precursors in vivo and that its downregulation during differentiation could be an important factor regulating the migratory phenotype of these cells.
Figures




References
-
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. - PubMed
-
- Albelda SM, Mette SA, Elder DE, Stewart R, Damjanovich L, Herlyn M, Buck C. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumour progression. Cancer Res. 1990;50:6757–6764. - PubMed
-
- Alfandari D, Whittaker CA, DeSimone DW, Darribere T. Integrin αv subunit is expressed on mesodermal cell surfaces during amphibian gastrulation. Dev Biol. 1995;170:249–261. - PubMed
-
- Almeida EAC, Huovila A-PJ, Sutherland AE, Stephens LE, Calarco PG, Shaw LM, Mercurio AM, Sonnenberg A, Primakoff P, Myles DG, White JM. Mouse egg integrin α6β1 functions as a sperm receptor. Cell. 1995;81:1095–1104. - PubMed
-
- Armstrong RC, Harvath L, Dubois-Dalcq ME. Type 1 astrocytes and oligodendrocyte-type 2 astrocyte glial progenitors migrate toward distinct molecules. J Neurosci Res. 1990;27:400–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
