An explanation for reflex blink hyperexcitability in Parkinson's disease. II. Nucleus raphe magnus
- PMID: 8929438
- PMCID: PMC6578942
- DOI: 10.1523/JNEUROSCI.16-22-07318.1996
An explanation for reflex blink hyperexcitability in Parkinson's disease. II. Nucleus raphe magnus
Abstract
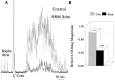
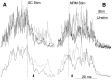
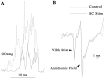
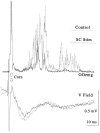
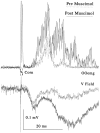
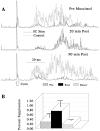
Hyperexcitable reflex blinks are a cardinal sign of Parkinson's disease. The first step in the circuit linking the basal ganglia and brainstem reflex blink circuits is the inhibitory nigrostriatal pathway (Basso et al., 1996). The current study reports the circuits linking the superior colliculus (SC) to trigeminal reflex blink circuits. Microstimulation of the deep layers of the SC suppresses subsequent reflex blinks at a latency of 5.4 msec. This microstimulation does not activate periaqueductal gray antinociceptive circuits. The brainstem structure linking SC to reflex blink circuits must suppress reflex blinks at a shorter latency than the SC and produce the same effect on reflex blink circuits as SC stimulation, and removal of the structure must block SC modulation of reflex blinks. Only the nucleus raphe magnus (NRM) meets these requirements. NRM microstimulation suppresses reflex blinks with a latency of 4.4 msec. Like SC stimulation, NRM microstimulation reduces the responsiveness of the spinal trigeminal nucleus. Finally, blocking the receptors for the NRM transmitter serotonin eliminates SC modulation of reflex blinks, and muscimol inactivation of the NRM transiently prevents SC modulation of reflex blinks. Thus, the circuit through which the basal ganglia modulates reflex blinking is (1) the substantia nigra pars reticulata inhibits SC neurons, (2) the SC excites tonically active NRM neurons, and (3) NRM neurons inhibit spinal trigeminal neurons involved in reflex blink circuits.
Figures




References
-
- Auerbach S, Fornal C, Jacobs B. Response of serotonin containing neurons in nucleus raphe magnus to morphine, noxious stimuli, and periaqueductal gray stimulation in freely moving cats. Exp Neurol. 1985;88:609–628. - PubMed
-
- Basso MA, Strecker RE, Evinger C. Midbrain 6-hydroxdopamine lesions modulate blink reflex excitability. Exp Brain Res. 1993;94:88–96. - PubMed
-
- Bronstein AM, Kennard C. Predictive ocular motor control in Parkinson’s disease. Brain. 1985;108:925–940. - PubMed
-
- Browne PA, Goldberg LJ. Direct evidence of presynaptic inhibition of a trigeminal reflex. Brain Res. 1978;143:369–372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
