A synaptic basis for memory storage in the cerebral cortex
- PMID: 8942956
- PMCID: PMC33630
- DOI: 10.1073/pnas.93.24.13453
A synaptic basis for memory storage in the cerebral cortex
Abstract
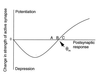
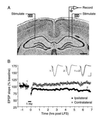
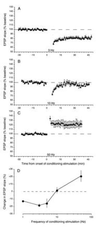
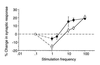
A cardinal feature of neurons in the cerebral cortex is stimulus selectivity, and experience-dependent shifts in selectivity are a common correlate of memory formation. We have used a theoretical "learning rule," devised to account for experience-dependent shifts in neuronal selectivity, to guide experiments on the elementary mechanisms of synaptic plasticity in hippocampus and neocortex. These experiments reveal that many synapses in hippocampus and neocortex are bidirectionally modifiable, that the modifications persist long enough to contribute to long-term memory storage, and that key variables governing the sign of synaptic plasticity are the amount of NMDA receptor activation and the recent history of cortical activity.
Figures


References
-
- Hebb D O. Organization of Behavior. New York: Wiley; 1949.
-
- Anderson J A, Rosenfeld E, editors. Neurocomputing: Foundations of Research. Cambridge, MA: MIT Press; 1988.
-
- Anderson J A, Mozer M C. In: Parallel Models of Associative Memory. Hinton G E, Anderson J A, editors. Hillsdale, NJ: Erlbaum; 1981.
-
- Wilson M, McNaughton B. Science. 1993;261:1055–1058. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

