Functional organization of the hippocampal memory system
- PMID: 8942963
- PMCID: PMC33637
- DOI: 10.1073/pnas.93.24.13500
Functional organization of the hippocampal memory system
Abstract
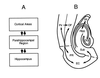
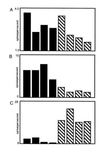
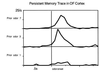
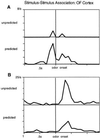
In humans declarative or explicit memory is supported by the hippocampus and related structures of the medial temporal lobe working in concert with the cerebral cortex. This paper reviews our progress in developing an animal model for studies of cortical-hippocampal interactions in memory processing. Our findings support the view that the cortex maintains various forms of memory representation and that hippocampal structures extend the persistence and mediate the organization of these codings. Specifically, the parahippocampal region, through direct and reciprocal interconnections with the cortex, is sufficient to support the convergence and extended persistence of cortical codings. The hippocampus itself is critical to the organization cortical representations in terms of relationships among items in memory and in the flexible memory expression that is the hallmark of declarative memory.
Figures

References
-
- Gray J A. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. New York: Oxford Univ. Press; 1982.
-
- Halgren E. In: Neuropsychology of Memory. Butters N, Squire L R, editors. New York: Guilford; 1984. pp. 165–182.
-
- Rolls E. In: Neural Models of Plasticity: Theoretical and Empirical Approaches. Byrne J H, Berry W O, editors. New York: Academic; 1989. pp. 240–265.
-
- Squire L R, Cohen N J, Nadel L. In: Memory Consolidation. Weingartner H, Parker E, editors. Hillsdale, NJ: Erlbaum; 1984. pp. 185–210.
-
- Teyler T J, DiScenna P. Behav Neurosci. 1986;100:147–154. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

