Zinc folds the N-terminal domain of HIV-1 integrase, promotes multimerization, and enhances catalytic activity
- PMID: 8942990
- PMCID: PMC19383
- DOI: 10.1073/pnas.93.24.13659
Zinc folds the N-terminal domain of HIV-1 integrase, promotes multimerization, and enhances catalytic activity
Abstract
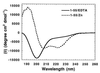
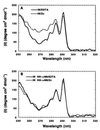
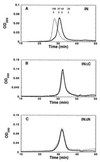
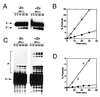
The N-terminal domain of HIV-1 integrase contains a pair of His and Cys residues (the HHCC motif) that are conserved among retroviral integrases. Although His and Cys residues are often involved in binding zinc, the HHCC motif does not correspond to any recognized class of zinc binding domain. We have investigated the binding of zinc to HIV-1 integrase protein and find that it binds zinc with a stoichiometry of one zinc per integrase monomer. Analysis of zinc binding to deletion derivatives of integrase locates the binding site to the N-terminal domain. Integrase with a mutation in the HHCC motif does not bind zinc, consistent with coordination of zinc by these residues. The isolated N-terminal domain is disordered in the absence of zinc but, in the presence of zinc, it adopts a secondary structure with a high alpha helical content. Integrase bound by zinc tetramerizes more readily than the apoenzyme and is also more active than the apoenzyme in in vitro integration assays. We conclude that binding of zinc to the HHCC motif stabilizes the folded state of the N-terminal domain of integrase and bound zinc is required for optimal enzymatic activity.
Figures

References
-
- Berg J M, Shi Y. Science. 1996;271:1081–1085. - PubMed
-
- Burke C J, Sanyal G, Bruner M W, Ryan J A, LaFemina R L, Robbins H L, Zeft A S, Middaugh C R, Cordingley M G. J Biol Chem. 1992;267:9639–9644. - PubMed
-
- Whitcomb J M, Hughes S H. Annu Rev Cell Biol. 1992;8:275–306. - PubMed
-
- Goff S P. Annu Rev Genet. 1992;26:527–544. - PubMed
-
- Vink C, Plasterk R H A. Trends Genet. 1993;9:433–438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

