Steroidogenic acute regulatory protein (StAR) retains activity in the absence of its mitochondrial import sequence: implications for the mechanism of StAR action
- PMID: 8943003
- PMCID: PMC19407
- DOI: 10.1073/pnas.93.24.13731
Steroidogenic acute regulatory protein (StAR) retains activity in the absence of its mitochondrial import sequence: implications for the mechanism of StAR action
Abstract
Steroidogenic acute regulatory protein (StAR) plays a critical role in steroid hormone biosynthesis, presumably by facilitating the delivery of cholesterol to P450scc in the inner mitochondrial membranes. StAR is synthesized as a 37-kDa preprotein that is processed to a 30-kDa mature form by cleavage of an N-terminal mitochondrial import sequence. To identify structural features required for StAR biological activity, we mutated the human StAR cDNA, including the deletion of N- and C-terminal sequences, and examined the ability of the mutants to promote steroidogenesis and enter the mitochondria of transfected COS-1 cells. Deletion of up to 62 residues from the N terminus (N-62) did not significantly affect steroidogenesis-enhancing activity. The N-terminal deletion mutants were associated with mitochondria-enriched fractions, but import and processing were progressively impaired with increasing length of the deletion. Immunogold electron microscopy and in vitro import assays showed that the active N-62 mutant was not imported into the mitochondria. Removal of the 28 C-terminal amino acids (C-28) inactivated StAR. Deletion of the C-terminal 10 amino acids (C-10) reduced steroidogenic activity by 53%, while truncation of the last 4 amino acids had no effect. The C-28 mutant StAR was not efficiently imported into mitochondria or processed, whereas some of the C-10 mutant was processed, indicating that import had occurred. We conclude that in the COS-1 cell system used, StAR does not need to enter into mitochondria to stimulate steroidogenesis and that residues in the C terminus are essential for steroidogenesis-enhancing activity. These findings imply that StAR acts via C-terminal domains on the outside of the mitochondria.
Figures




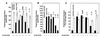
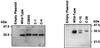

Comment in
-
Twinkle, twinkle little StAR, how we wonder what you are.Proc Natl Acad Sci U S A. 1996 Nov 26;93(24):13552-4. doi: 10.1073/pnas.93.24.13552. Proc Natl Acad Sci U S A. 1996. PMID: 8942971 Free PMC article. Review. No abstract available.
References
-
- Lin D, Sugawara T, Strauss J F, III, Clark B J, Stocco D M, Saenger P, Rogol A, Miller W L. Science. 1995;267:1828–1831. - PubMed
-
- Stocco D M, Clark B J. Endocr Rev. 1996;17:221–244. - PubMed
-
- Pon L A, Hartigan J A, Orme-Johnson N R. J Biol Chem. 1986;261:13309–13316. - PubMed
-
- Epstein L F, Orme-Johnson N. J Biol Chem. 1991;266:19739–19745. - PubMed
-
- Clark B J, Wells J, King S R, Stocco D M. J Biol Chem. 1994;269:28314–28322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

