Inhibition of interleukin 1 beta-converting enzyme-mediated apoptosis of mammalian cells by baculovirus IAP
- PMID: 8943013
- PMCID: PMC19426
- DOI: 10.1073/pnas.93.24.13786
Inhibition of interleukin 1 beta-converting enzyme-mediated apoptosis of mammalian cells by baculovirus IAP
Abstract
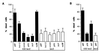
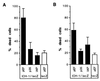
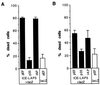
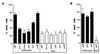
Apoptosis can be a potent weapon against viral infection and consequently has selected for viruses carrying antiapoptosis genes. Two baculovirus proteins, IAP and p35, can prevent insect cells from dying in response to infection. p35, which interferes with members of the Ced-3 family of cysteine proteases, can also function in mammalian cells. We investigated the ability of IAP from Orgyia pseudotsugata nuclear polyhedrosis virus to prevent death of mammalian cells. IAP was transiently expressed in mammalian cells and its ability to block cell death caused by expression of interleukin-1 beta converting enzyme (ICE), FADD, or the ICE homologues ICH-1 and ICE-Lap3, was investigated. IAP strongly inhibited ICE- and ICH-1-induced cell death but protected only partially against death by overexpression of FADD and not at all against death due to enforced ICE-Lap3 expression. These results demonstrate that a baculoviral IAP protein can functionally interact with conserved components of the apoptosis machinery in mammalian cells.
Figures

References
-
- Vaux D L, Häcker G, Strasser A. Cell. 1994;76:777–779. - PubMed
-
- Steller H. Science. 1995;267:1445–1462. - PubMed
-
- Kumar S. Trends Biochem Sci. 1995;20:198–202. - PubMed
-
- Yuan J, Shaham S, Ledoux S, Ellis H M, Horvitz H R. Cell. 1993;75:641–652. - PubMed
-
- Miura M, Zhu H, Rotello R, Hartweig E A, Yuan J. Cell. 1993;75:653–660. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

