The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex
- PMID: 8943045
- PMCID: PMC19479
- DOI: 10.1073/pnas.93.24.13973
The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex
Abstract
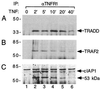
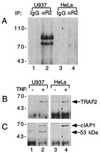
The two cell surface receptors for tumor necrosis factor (TNF) interact with a number of intracellular signal transducing proteins. The association of TRADD, a 34-kDa cytoplasmic protein containing a C-terminal death domain, with aggregated TNF receptor 1 (TNF-R1) through their respective death domains leads to NF-kappa B activation and programmed cell death. In contrast, TNF receptor 2 (TNF-R2) interacts with the TNF receptor associated factors 2/1 (TRAF2/TRAF1) heterocomplex, which mediates the recruitment of two cellular inhibitor of apoptosis proteins (c-IAP1 and c-IAP2) to TNF-R2. Here we show that the TNF-R2 signal transducers TRAF2 and c-IAP1 are a part of the TNF-R1 signaling complex. The recruitment of TRAF2 and c-IAP1 to TNF-R1 is TNF-dependent, is mediated by TRADD, and is independent of TNF-R2. These data establish the physiological involvement of TRAF2 and c-IAP1 in TNF-R1 signaling and help provide a molecular explanation for both the overlapping and distinct signals generated by the two TNF receptors.
Figures


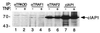

References
-
- Goeddel D V, Aggarwal B B, Gray P W, Leung D W, Nedwin G E, Palladino M A, Patton J S, Pennica D, Shepard H M, Sugarman B J, Wong G H W. Cold Spring Harbor Symp Quant Biol. 1986;51:597–609. - PubMed
-
- Beutler B, Cerami A. Annu Rev Biochem. 1988;57:505–518. - PubMed
-
- Fiers W. FEBS Lett. 1991;285:199–212. - PubMed
-
- Loetscher H, Pan Y-C E, Lahm H W, Gentz R, Brockhaus M, Tabuch H, Lesslauer W. Cell. 1990;61:351–359. - PubMed
-
- Schall T J, Lewis M, Koller K J, Lee A, Rice G C, Wong G H W, Gatanaga T, Granger G A, Lentz R, Raab H, Kohr W J, Goeddel D V. Cell. 1990;61:361–370. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

