Brain drug delivery of small molecules using immunoliposomes
- PMID: 8943078
- PMCID: PMC19511
- DOI: 10.1073/pnas.93.24.14164
Brain drug delivery of small molecules using immunoliposomes
Abstract
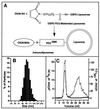
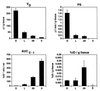
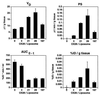
Immunoliposomes (antibody-directed liposomes) were used in the present study for delivery of the antineoplastic agent daunomycin to the rat brain. A coupling procedure was introduced, which allows conjugation of a thiolated antibody to maleimide-grafted 85-nm liposomes sterically stabilized with PEG. Antibody was thereby coupled to the terminal end of a PEG-conjugated linker lipid. No brain uptake of PEG-conjugated liposomes carrying [3H]daunomycin was observed. However, brain targeting of immunoliposomes carrying [3H]daunomycin was mediated by the OX26 monoclonal antibody to the rat transferrin receptor, which is selectively enriched at the brain microvascular endothelium that comprises the blood-brain barrier in vivo. Coupling of 30 OX26 antibodies per liposome resulted in optimal brain delivery. Saturation of delivery was observed at higher antibody densities. Determination of brain levels of immunoliposomes over 24 h revealed that immunoliposomes accumulate in brain tissue. Brain targeting of immunoliposomes was not observed in immunoliposomes conjugated with a mouse IgG2a isotype control. In addition, coinjection of free OX26 saturated plasma clearance of immunoliposomes. Since a single liposome may carry > or = 10,000 drug molecules, the use of PEG-conjugated immunoliposomes increases the drug carrying capacity of the monoclonal antibody by up to 4 logarithmic orders in magnitude. In summary, specific OX26-mediated targeting of daunomycin to the rat brain was achieved by the use of an immunoliposome-based drug delivery system.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

