Symmetry arguments in chemistry
- PMID: 8962036
- PMCID: PMC34471
- DOI: 10.1073/pnas.93.25.14260
Symmetry arguments in chemistry
Abstract
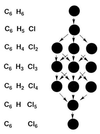
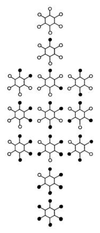
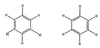
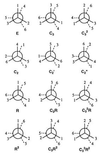
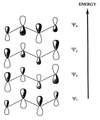
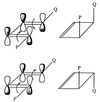
The use (and misuse) of symmetry arguments in constructing molecular models and in the interpretation of experimental observations bearing on molecular structure (spectroscopy, diffraction, etc.) is discussed. Examples include the development of point groups and space groups for describing the external and internal symmetry of crystals, the derivation of molecular symmetry by counting isomers (the benzene structure), molecular chirality, the connection between macroscopic and molecular chirality, pseudorotation, the symmetry group of nonrigid molecules, and the use of orbital symmetry arguments in discussing aspects of chemical reactivity.
Figures
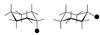
References
-
- Kepler, J. (1611) De Nive Hexangula: On the Six-Cornered Snowflake, translated by Hardie, C. (1966) (Clarendon Press, Oxford, U.K.).
-
- Haüy R J. Essai d’une théorie sur la structure des cristaux appliqué a plusieurs genres de substances cristallines. Paris: Gogué & Née de la Rochelle; 1784.
-
- Hessel, J. F. C. (1830) Kristallometrie oder Kristallonomie und Kristallographie. Reprinted in Ostwald’s Klassiker der exakten Wissenschaften (1897) (Engelmann, Leipzig).
-
- Hilton H. Mathematical Crystallography and the Theory of Groups of Movements. Oxford, UK; reprinted 1963 Dover Books, New York: Clarendon; 1903.
MeSH terms
LinkOut - more resources
Full Text Sources

