The chemistry of the S-nitrosoglutathione/glutathione system
- PMID: 8962068
- PMCID: PMC26149
- DOI: 10.1073/pnas.93.25.14428
The chemistry of the S-nitrosoglutathione/glutathione system
Abstract
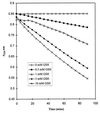
S-Nitrosothiols have generated considerable interest due to their ability to act as nitric oxide (NO) donors and due to their possible involvement in bioregulatory systems-e.g., NO transfer reactions. Elucidation of the reaction pathways involved in the modification of the thiol group by S-nitrosothiols is important for understanding the role of S-nitroso compounds in vivo. The modification of glutathione (GSH) in the presence of S-nitrosoglutathione (GSNO) was examined as a model reaction. Incubation of GSNO (1 mM) with GSH at various concentrations (1-10 mM) in phosphate buffer (pH 7.4) yielded oxidized glutathione, nitrite, nitrous oxide, and ammonia as end products. The product yields were dependent on the concentrations of GSH and oxygen. Transient signals corresponding to GSH conjugates, which increased by one mass unit when the reaction was carried out with 15N-labeled GSNO, were identified by electrospray ionization mass spectrometry. When morpholine was present in the reaction system, N-nitrosomorpholine was formed. Increasing concentrations of either phosphate or GSH led to lower yields of N-nitrosomorpholine. The inhibitory effect of phosphate may be due to reaction with the nitrosating agent, nitrous anhydride (N2O3), formed by oxidation of NO. This supports the release of NO during the reaction of GSNO with GSH. The products noted above account quantitatively for virtually all of the GSNO nitrogen consumed during the reaction, and it is now possible to construct a complete set of pathways for the complex transformations arising from GSNO + GSH.
Figures

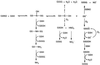
References
-
- Josephy P D, Rehorek D, Janzen E G. Tetrahedron Lett. 1984;25:1685–1688.
-
- Williams D L H. Chem Soc Rev. 1985;14:171–196.
-
- Kelm M, Schrader J. Circ Res. 1990;66:1561–1575. - PubMed
-
- Oae S, Shinhama K. Org Prep Proced Int. 1983;15:165–198.
-
- Kowaluk E A, Fung H L. J Pharmacol Exp Ther. 1990;255:1256–1264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

