Ordered water molecules as key allosteric mediators in a cooperative dimeric hemoglobin
- PMID: 8962085
- PMCID: PMC26166
- DOI: 10.1073/pnas.93.25.14526
Ordered water molecules as key allosteric mediators in a cooperative dimeric hemoglobin
Abstract
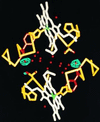
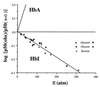
One of the most remarkable structural aspects of Scapharca dimeric hemoglobin is the disruption of a very well-ordered water cluster at the subunit interface upon ligand binding. We have explored the role of these crystallographically observed water molecules by site-directed mutagenesis and osmotic stress techniques. The isosteric mutation of Thr-72-->Val in the interface increases oxygen affinity more than 40-fold with a surprising enhancement of cooperativity. The only significant structural effect of this mutation is to destabilize two ordered water molecules in the deoxy interface. Wild-type Scapharca hemoglobin is strongly sensitive to osmotic conditions. Upon addition of glycerol, striking changes in Raman spectrum of the deoxy form are observed that indicate a transition toward the liganded form. Increased osmotic pressure, which lowers the oxygen affinity in human hemoglobin, raises the oxygen affinity of Scapharca hemoglobin regardless of whether the solute is glycerol, glucose, or sucrose. Analysis of these results provides an estimate of six water molecules lost upon oxygen binding to the dimer, in good agreement with eight predicted from crystal structures. These experiments suggest that the observed cluster of interfacial water molecules plays a crucial role in communication between subunits.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

